Abstract
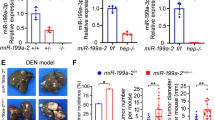
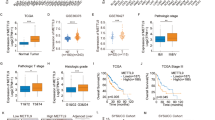
Advanced-stage hepatocellular carcinoma (HCC) remains an untreatable disease with an overall survival of less than one year. One of the critical molecular mediators contributing to increased resistance to therapy and relapse, is increased hypoxia-inducible factor 1α (HIF-1α) levels, leading to metastasis of tumor cells. Several microRNAs are known to be dysregulated and impact HIF-1α expression in HCC. An in silico analysis demonstrated that hsa-miR-199a-5p is downregulated at various stages of HCC and is known to repress HIF-1α expression. Based on this analysis, we developed a combinatorial suicide gene therapy by employing hepatotropic Adeno-associated virus-based vectors encoding an inducible caspase 9 (iCasp9) and miR-199a. The overexpression of miR-199a-5p alone significantly decreased ( ~ 2-fold vs. Mock treated cells, p < 0.05) HIF-1α mRNA levels, with a concomitant increase in cancer cell cytotoxicity in Huh7 cells in vitro and in xenograft models in vivo. To further enhance the efficacy of gene therapy, we evaluated the synergistic therapeutic effect of AAV8-miR-199a and AAV6-iCasp9 in a xenograft model of HCC. Our data revealed that mice receiving combination suicide gene therapy exhibited reduced expression of HIF-1α ( ~ 4-fold vs. Mock, p < 0.001), with a significant reduction in tumor growth when compared to mock-treated animals. These findings underscore the therapeutic potential of downregulating HIF-1α during suicide gene therapy for HCC.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data generated in this study is available upon reasonable request to the corresponding author.
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63.
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Prim. 2021;7:6.
Yuen VW, Wong CC. Hypoxia-inducible factors and innate immunity in liver cancer. J Clin Invest. 2020;130:5052–62.
Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430–9.
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54.
Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402.
Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–4.
Wigerup C, Pahlman S, Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharm Ther. 2016;164:152–69.
Walsh JC, Lebedev A, Aten E, Madsen K, Marciano L, Kolb HC. The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid Redox Signal. 2014;21:1516–54.
Phillips RM, Hendriks HR, Peters GJ, Pharmacology E. Molecular Mechanism G. EO9 (Apaziquone): from the clinic to the laboratory and back again. Br J Pharm. 2013;168:11–18.
Singh V, Khan N, Jayandharan GR. Vector engineering, strategies and targets in cancer gene therapy. Cancer Gene Ther. 2022;29:402–17.
Santiago-Ortiz JL, Schaffer DV. Adeno-associated virus (AAV) vectors in cancer gene therapy. J Control Release. 2016;240:287–301.
Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G. Viral vector platforms within the gene therapy landscape. Signal Transduct Target Ther. 2021;6:53.
Sun X, Jiang H, Jiang X, Tan H, Meng Q, Sun B, et al. Antisense hypoxia-inducible factor-1alpha augments transcatheter arterial embolization in the treatment of hepatocellular carcinomas in rats. Hum Gene Ther. 2009;20:314–24.
Liu F, Wang P, Jiang X, Tan G, Qiao H, Jiang H, et al. Antisense hypoxia-inducible factor 1alpha gene therapy enhances the therapeutic efficacy of doxorubicin to combat hepatocellular carcinoma. Cancer Sci. 2008;99:2055–61.
Ma S, Sun J, Guo Y, Zhang P, Liu Y, Zheng D, et al. Combination of AAV-TRAIL with miR-221-Zip Therapeutic Strategy Overcomes the Resistance to TRAIL Induced Apoptosis in Liver Cancer. Theranostics. 2017;7:3228–42.
Khan N, Bammidi S, Chattopadhyay S, Jayandharan GR. Combination Suicide Gene Delivery with an Adeno-Associated Virus Vector Encoding Inducible Caspase-9 and a Chemical Inducer of Dimerization Is Effective in a Xenotransplantation Model of Hepatocellular Carcinoma. Bioconjug Chem. 2019;30:1754–62.
Khan N, Maurya S, Bammidi S, Jayandharan GR. AAV6 Vexosomes Mediate Robust Suicide Gene Delivery in a Murine Model of Hepatocellular Carcinoma. Mol Ther Methods Clin Dev. 2020;17:497–504.
Otmani K, Lewalle P. Tumor Suppressor miRNA in Cancer Cells and the Tumor Microenvironment: Mechanism of Deregulation and Clinical Implications. Front Oncol. 2021;11:708765.
Raue R, Frank AC, Syed SN, Brune B Therapeutic Targeting of MicroRNAs in the Tumor Microenvironment. Int J Mol Sci. 2021;22.
Shu J, Xia Z, Li L, Liang ET, Slipek N, Shen D, et al. Dose-dependent differential mRNA target selection and regulation by let-7a-7f and miR-17-92 cluster microRNAs. RNA Biol. 2012;9:1275–87.
Diener C, Keller A, Meese E. Emerging concepts of miRNA therapeutics: from cells to clinic. Trends Genet. 2022;38:613–26.
Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–17.
Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong Q, et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell. 2011;19:232–43.
Yin L, Keeler GD, Zhang Y, Hoffman BE, Ling C, Qing K, et al. AAV3-miRNA vectors for growth suppression of human hepatocellular carcinoma cells in vitro and human liver tumors in a murine xenograft model in vivo. Gene Ther. 2021;28:422–34.
Xu H, Zhao L, Fang Q, Sun J, Zhang S, Zhan C, et al. MiR-338-3p inhibits hepatocarcinoma cells and sensitizes these cells to sorafenib by targeting hypoxia-induced factor 1alpha. PLoS One. 2014;9:e115565.
Wang C, Song B, Song W, Liu J, Sun A, Wu D, et al. Underexpressed microRNA-199b-5p targets hypoxia-inducible factor-1alpha in hepatocellular carcinoma and predicts prognosis of hepatocellular carcinoma patients. J Gastroenterol Hepatol. 2011;26:1630–7.
Agarwal V, Bell GW, Nam JW, Bartel DP Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4.
Paraskevopoulou MD, Georgakilas G, Kostoulas N, Vlachos IS, Vergoulis T, Reczko M, et al. DIANA-microT web server v5.0: service integration into miRNA functional analysis workflows. Nucleic Acids Res. 2013;41:W169–W173.
Chen Y, Wang X. miRDB: an online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020;48:D127–D131.
Vejnar CE, Zdobnov EM. MiRmap: comprehensive prediction of microRNA target repression strength. Nucleic Acids Res. 2012;40:11673–83.
Huang HY, Lin YC, Cui S, Huang Y, Tang Y, Xu J, et al. miRTarBase update 2022: an informative resource for experimentally validated miRNA-target interactions. Nucleic Acids Res. 2022;50:D222–D30.
Kavakiotis I, Alexiou A, Tastsoglou S, Vlachos IS, Hatzigeorgiou AG. DIANA-miTED: a microRNA tissue expression database. Nucleic Acids Res. 2022;50:D1055–D61.
Chandrashekar DS, Karthikeyan SK, Korla PK, Patel H, Shovon AR, Athar M, et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia. 2022;25:18–27.
Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47:W556–W60.
Menyhart O, Nagy A, Gyorffy B. Determining consistent prognostic biomarkers of overall survival and vascular invasion in hepatocellular carcinoma. R Soc Open Sci. 2018;5:181006.
Mary B, Maurya S, Arumugam S, Kumar V, Jayandharan GR. Post-translational modifications in capsid proteins of recombinant adeno-associated virus (AAV) 1-rh10 serotypes. FEBS J. 2019;286:4964–81.
Singh V, Pathak S, Kumar N, Jayandharan GR. Development of an Optimized Promoter System for Exosomal and Naked AAV Vector-Based Suicide Gene Therapy in Hepatocellular Carcinoma. ACS Omega. 2024;9:30945–53.
Kyrylkova K, Kyryachenko S, Leid M, Kioussi C. Detection of apoptosis by TUNEL assay. Methods Mol Biol. 2012;887:41–7.
Cancer Genome Atlas Research Network. Electronic address wbe, Cancer Genome Atlas Research N. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 2017;169:1327–41.e23.
Liu P, Xia P, Fu Q, Liu C, Luo Q, Cheng L, et al. miR-199a-5p inhibits the proliferation of hepatocellular carcinoma cells by regulating CDC25A to induce cell cycle arrest. Biochem Biophys Res Commun. 2021;571:96–103.
Li B, He L, Zuo D, He W, Wang Y, Zhang Y, et al. Mutual Regulation of MiR-199a-5p and HIF-1alpha Modulates the Warburg Effect in Hepatocellular Carcinoma. J Cancer. 2017;8:940–9.
Zheng X, Liu X, Lei Y, Wang G, Liu M. Glypican-3: A Novel and Promising Target for the Treatment of Hepatocellular Carcinoma. Front Oncol. 2022;12:824208.
Pathak S, Singh V, Kumar N, Jayandharan GR. Inducible caspase 9-mediated suicide gene therapy using AAV6 vectors in a murine model of breast cancer. Mol Ther Methods Clin Dev. 2023;31:101166.
Zhan Y, Zheng N, Teng F, Bao L, Liu F, Zhang M, et al. MiR-199a/b-5p inhibits hepatocellular carcinoma progression by post-transcriptionally suppressing ROCK1. Oncotarget. 2017;8:67169–80.
Yamada S, Utsunomiya T, Morine Y, Imura S, Ikemoto T, Arakawa Y, et al. Expressions of hypoxia-inducible factor-1 and epithelial cell adhesion molecule are linked with aggressive local recurrence of hepatocellular carcinoma after radiofrequency ablation therapy. Ann Surg Oncol. 2014;21:S436–42.
Yuan G, Zeng CL, Zhu DD, Shi XJ. Influences of RFA combined with TACE on the HIF-1alpha and EGR level of patients with primary hepatic carcinoma. Eur Rev Med Pharm Sci. 2017;21:1738–45.
Chen Y, Bei J, Liu M, Huang J, Xie L, Huang W, et al. Sublethal heat stress-induced O-GlcNAcylation coordinates the Warburg effect to promote hepatocellular carcinoma recurrence and metastasis after thermal ablation. Cancer Lett. 2021;518:23–34.
Wu L, Zhang YS, Ye ML, Shen F, Liu W, Hu HS, et al. Overexpression and correlation of HIF-2alpha, VEGFA and EphA2 in residual hepatocellular carcinoma following high-intensity focused ultrasound treatment: Implications for tumor recurrence and progression. Exp Ther Med. 2017;13:3529–34.
Mendez-Blanco C, Fondevila F, Garcia-Palomo A, Gonzalez-Gallego J, Mauriz JL. Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp Mol Med. 2018;50:1–9.
Zeng Z, Lu Q, Liu Y, Zhao J, Zhang Q, Hu L, et al. Effect of the Hypoxia Inducible Factor on Sorafenib Resistance of Hepatocellular Carcinoma. Front Oncol. 2021;11:641522.
Yang X, Zheng Y, Tan J, Tian R, Shen P, Cai W, et al. MiR-199a-5p-HIF-1alpha-STAT3 Positive Feedback Loop Contributes to the Progression of Non-Small Cell Lung Cancer. Front Cell Dev Biol. 2020;8:620615.
Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN, et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–61.
Dhungel B, Andrzejewski S, Jayachandran A, Shrestha R, Ramlogan-Steel CA, Layton CJ, et al. Evaluation of the Glypican 3 promoter for transcriptional targeting of hepatocellular carcinoma. Gene Ther. 2018;25:115–28.
Montano-Samaniego M, Bravo-Estupinan DM, Mendez-Guerrero O, Alarcon-Hernandez E, Ibanez-Hernandez M. Strategies for Targeting Gene Therapy in Cancer Cells With Tumor-Specific Promoters. Front Oncol. 2020;10:605380.
Marabelle A, Tselikas L, de Baere T, Houot R. Intratumoral immunotherapy: using the tumor as the remedy. Ann Oncol. 2017;28:xii33–xii43.
Zeng LiZB, Chen ZJ, Luo Q, Hu SQ. WX. Recombinant AAV-mediated HSVtk gene transfer with direct intratumoral injections and Tet-On regulation for implanted human breast cancer. BMC Cancer. 2006;6:66.
Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21:808–20.
Kudo M. Durvalumab plus tremelimumab in unresectable hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2022;11:592–6.
Brown ZJ, Heinrich B, Greten TF. Mouse models of hepatocellular carcinoma: an overview and highlights for immunotherapy research. Nat Rev Gastroenterol Hepatol. 2018;15:536–54.
Acknowledgements
We thank the central experimental animal facility, IIT-Kanpur, for housing the animals and cryosectioning facility. We acknowledge the assistance provided by Live Cell Imaging Lab, ACMS, and IIT Kanpur for confocal imaging. We also thank Dr. Saraswat Pathology, Kanpur, for preparing the paraffin sections and H&E staining of tumor samples. We thank Ms. Pratiksha Sarangi and Ms. Rupam Ashok Singh for technical help.
Funding
We acknowledge IIT Kanpur for providing intramural funding for this research work. S.P. and V.S. received study fellowships from the Human Resource Development Group, Council of Scientific & Industrial Research, and the Ministry of Human Resource Development, Government of India, respectively.
Author information
Authors and Affiliations
Contributions
SP, VS, and NK performed the experiments; SP and GRJ interpreted the data and wrote the manuscript; GRJ conceptualized and designed the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare that IIT Kanpur has applied for patents on AAV vectors, and some of these have been licensed.
Ethical approval
All methods were performed in accordance with the relevant guidelines and regulations. Experiments related to recombinant DNA vectors and the production of AAV vectors have been approved by the Institute Bio-Safety Committee (IBSC), IIT Kanpur, India (Registration no. INDI14102019728). All animal-related experiments were conducted following approval from the Institute Animal Ethics Committee (IAEC), IIT Kanpur, India (Registration no. 810/GO/ReBi/S/2003/CPCSEA).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pathak, S., Singh, V., Kumar G., N. et al. AAV-mediated combination gene therapy of inducible Caspase 9 and miR-199a-5p is therapeutic in hepatocellular carcinoma. Cancer Gene Ther 31, 1796–1803 (2024). https://doi.org/10.1038/s41417-024-00844-x
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41417-024-00844-x