Abstract
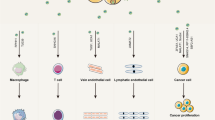
Tumor metastasis regulated by multiple complicated pathways is closely related to variations in the tumor microenvironment (TME). Exosomes can regulate the TME through various mechanisms. Exosomes derived from tumor cells carry a variety of substances, including long non-coding RNAs (lncRNAs), play important roles in intercellular communication and act as critical determinants influencing tumor metastasis. In this review, we elaborate on several pivotal processes through which lncRNAs regulate tumor metastasis, including the regulation of epithelial‒mesenchymal transition, promotion of angiogenesis and lymphangiogenesis, enhancement of the stemness of tumor cells, and evasion of immune clearance. Additionally, we comprehensively summarized a diverse array of potential tumor-derived exosomal lncRNA biomarkers to facilitate accurate diagnosis and prognosis in a clinical setting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
24 April 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41417-025-00909-5
References
Gravis G, Boher JM, Joly F, Soulie M, Albiges L, Priou F, et al. Androgen Deprivation Therapy (ADT) Plus Docetaxel Versus ADT Alone in Metastatic Non castrate Prostate Cancer: Impact of Metastatic Burden and Long-term Survival Analysis of the Randomized Phase 3 GETUG-AFU15 Trial. Eur Urol. 2016;70:256–62. https://doi.org/10.1016/j.eururo.2015.11.005.
Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393:2051–8. https://doi.org/10.1016/s0140-6736(18)32487-5.
Liu SJ, Dang HX, Lim DA, Feng FY, Maher CA. Long noncoding RNAs in cancer metastasis. Nat Rev Cancer. 2021;21:446–60. https://doi.org/10.1038/s41568-021-00353-1.
Li F, Xu T, Chen P, Sun R, Li C, Zhao X, et al. Platelet-derived extracellular vesicles inhibit ferroptosis and promote distant metastasis of nasopharyngeal carcinoma by upregulating ITGB3. Int J Biol Sci. 2022;18:5858–72. https://doi.org/10.7150/ijbs.76162.
Smid M, Wang YX, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68:3108–14. https://doi.org/10.1158/0008-5472.CAN-07-5644.
Davis RT, Blake K, Ma D, Gabra MBI, Hernandez GA, Phung AT, et al. Transcriptional diversity and bioenergetic shift in human breast cancer metastasis revealed by single-cell RNA sequencing. Nat Cell Biol. 2020;22:310–20. https://doi.org/10.1038/s41556-020-0477-0.
Liu YM, Ge JY, Chen YF, Liu T, Chen L, Liu CC, et al. Combined Single-Cell and Spatial Transcriptomics Reveal the Metabolic Evolvement of Breast Cancer during Early Dissemination. Adv Sci. 2023;10:e2205395. https://doi.org/10.1002/advs.202205395.
Dashzeveg NK, Jia Y, Zhang Y, Gerratana L, Patel P, Shajahan A, et al. Dynamic Glycoprotein Hyposialylation Promotes Chemotherapy Evasion and Metastatic Seeding of Quiescent Circulating Tumor Cell Clusters in Breast Cancer. Cancer Discov. 2023;13:2050–71. https://doi.org/10.1158/2159-8290.CD-22-0644.
Ma L, Wang L, Chang C-W, Heinrich S, Dominguez D, Forgues M, et al. Single-cell atlas of tumor clonal evolution in liver cancer. 2020. https://doi.org/10.1101/2020.08.18.254748.
Choi DS, Kim DK, Kim YK, Gho YS. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013;13:1554–71. https://doi.org/10.1002/pmic.201200329.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367. https://doi.org/10.1126/science.aau6977.
Han QF, Li WJ, Hu KS, Gao J, Zhai WL, Yang JH, et al. Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer. Mol Cancer. 2022;21:207. https://doi.org/10.1186/s12943-022-01671-0.
Isaac R, Reis FCG, Ying W, Olefsky JM. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021;33:1744–62. https://doi.org/10.1016/j.cmet.2021.08.006.
Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684–707. https://doi.org/10.7150/thno.41580.
Nakamura K, Zhu Z, Roy S, Jun E, Han H, Munoz RM, et al. An Exosome-based Transcriptomic Signature for Noninvasive, Early Detection of Patients With Pancreatic Ductal Adenocarcinoma: A Multicenter Cohort Study. Gastroenterology. 2022;163:1252–66.e2. https://doi.org/10.1053/j.gastro.2022.06.090.
Zhou BT, Xu KL, Zheng X, Chen T, Wang J, Song Y, et al. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct Target Ther. 2020;5:144. https://doi.org/10.1038/s41392-020-00258-9.
Wu P, Mo YZ, Peng M, Tang T, Zhong Y, Deng X, et al. Emerging role of tumor-related functional peptides encoded by lncRNA and circRNA. Mol Cancer. 2020;19:22. https://doi.org/10.1186/s12943-020-1147-3.
Tsai MC, Manor O, Wan Y, Mosammaparast N. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–93.
He Y, Jiang X, Duan L, Xiong Q, Yuan Y, Liu P, et al. LncRNA PKMYT1AR promotes cancer stem cell maintenance in non-small cell lung cancer via activating Wnt signaling pathway. Molecular Cancer. 2021;20. https://doi.org/10.1186/s12943-021-01469-6.
Ransohoff JD, Wei YN, Khavari PA. The functions and unique features of long intergenic non-coding RNA. Nat Rev Mol Cell Biol. 2018;19:143–57. https://doi.org/10.1038/nrm.2017.104.
Popadin K, Gutierrez-Arcelus M, Dermitzakis ET, Antonarakis SE. Genetic and epigenetic regulation of human lincRNA gene expression. Am J Hum Genet. 2013;93:1015–26. https://doi.org/10.1016/j.ajhg.2013.10.022.
Herman AB, Tsitsipatis D, Gorospe M. Integrated lncRNA function upon genomic and epigenomic regulation. Mol Cell. 2022;82:2252–66. https://doi.org/10.1016/j.molcel.2022.05.027.
Palazzo AF, Koonin EV. Functional Long Non-coding RNAs Evolve from Junk Transcripts. Cell. 2020;183:1151–61. https://doi.org/10.1016/j.cell.2020.09.047.
Jiang QH, Wang JX, Wu XL, Ma R, Zhang T, Jin S, et al. LncRNA2Target: a database for differentially expressed genes after lncRNA knockdown or overexpression. Nucleic Acids Res. 2015;43:193–6. https://doi.org/10.1093/nar/gku1173.
Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. https://doi.org/10.1146/annurev-cellbio-101512-122326.
McCabe EM, Rasmussen TP. lncRNA involvement in cancer stem cell function and epithelial-mesenchymal transitions. Semin Cancer Biol. 2021;75:38–48. https://doi.org/10.1016/j.semcancer.2020.12.012.
Robinson EK, Covarrubias S, Carpenter S. The how and why of lncRNA function: An innate immune perspective. Biochim Biophys Acta Gene Regul Mech. 2020;1863:194419. https://doi.org/10.1016/j.bbagrm.2019.194419.
Zong Y, Wang X, Cui B, Xiong X, Wu A, Lin C, et al. Decoding the regulatory roles of non-coding RNAs in cellular metabolism and disease. Mol Ther. 2023;31:1562–76. https://doi.org/10.1016/j.ymthe.2023.04.012.
Yang Z, Jiang S, Shang JJ, Jiang Y, Dai Y, Xu B, et al. LncRNA: Shedding light on mechanisms and opportunities in fibrosis and aging. Ageing Res Rev. 2019;52:17–31. https://doi.org/10.1016/j.arr.2019.04.001.
Ma YX, Zhang J, Wen LX, Lin AF. Membrane-lipid associated lncRNA: A new regulator in cancer signaling. Cancer Lett. 2018;419:27–9. https://doi.org/10.1016/j.canlet.2018.01.008.
Li SL, Li YC, Chen B, Zhao J, Yu S, Tang Y, et al. exoRBase: a database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res. 2018;46:D106–D12. https://doi.org/10.1093/nar/gkx891.
Nederveen JP, Warnier G, Di Carlo A, Nilsson MI, Tarnopolsky MA. Extracellular Vesicles and Exosomes: Insights From Exercise Science. Front Physiol. 2020;11:604274. https://doi.org/10.3389/fphys.2020.604274.
Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–88. https://doi.org/10.1007/s00018-011-0689-3.
Krylova SV, Feng D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int J Mol Sci. 2023;24. https://doi.org/10.3390/ijms24021337.
Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–41. https://doi.org/10.1016/j.cell.2009.02.006.
Conigliaro A, Costa V, Lo Dico A, Saieva L, Buccheri S, Dieli F, et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer. 2015;14:155. https://doi.org/10.1186/s12943-015-0426-x.
Karamanos NK, Theocharis AD, Piperigkou Z, Manou D, Passi A, Skandalis SS, et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021;288:6850–912. https://doi.org/10.1111/febs.15776.
Yuan Z, Li Y, Zhang S, Wang X, Dou H, Yu X, et al. Extracellular matrix remodeling in tumor progression and immune escape: from mechanisms to treatments. Mol Cancer. 2023;22:48. https://doi.org/10.1186/s12943-023-01744-8.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–98. https://doi.org/10.1038/nrc.2016.73.
Tsuji T, Ibaragi S, Shima K, Hu MG, Katsurano M, Sasaki A, et al. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res. 2008;68:10377–86. https://doi.org/10.1158/0008-5472.CAN-08-1444.
Yilmaz M, Christofori G. Mechanisms of motility in metastasizing cells. Mol Cancer Res. 2010;8:629–42. https://doi.org/10.1158/1541-7786.MCR-10-0139.
Zhao Y, Guo S, Deng J, Shen J, Du F, Wu X, et al. VEGF/VEGFR-Targeted Therapy and Immunotherapy in Non-small Cell Lung Cancer: Targeting the Tumor Microenvironment. Int J Biol Sci. 2022;18:3845–58. https://doi.org/10.7150/ijbs.70958.
Zeng ZC, Li YL, Pan YJ, Lan X, Song F, Sun J, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9:5395. https://doi.org/10.1038/s41467-018-07810-w.
Karaman S, Detmar M. Mechanisms of lymphatic metastasis. J Clin Invest. 2014;124:922–8. https://doi.org/10.1172/JCI71606.
Yang EL, Wang X, Gong ZY, Yu M, Wu H, Zhang D. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Target Ther. 2020;5:242. https://doi.org/10.1038/s41392-020-00359-5.
Marin-Valencia I, Yang C, Mashimo T, Cho S, Baek H, Yang XL, et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012;15:827–37. https://doi.org/10.1016/j.cmet.2012.05.001.
Guppy M, Leedman P, Zu XL. V R. Contribution by different fuels and metabolic pathways to the total ATP turnover of proliferating MCF-7 breast cancer cells. Biochem J. 2002;364:309–15.
Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–64. https://doi.org/10.1146/annurev-cellbio-092910-154237.
Enzo E, Santinon G, Pocaterra A, Aragona M, Forcato M, Bresolin S, et al. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J. 2015;34:1349–470. https://doi.org/10.15252/embj.201490379.
Wang M, Wang N, Zhang H, Sun L, Xu Q, Liang L, et al. Fatty acid transport protein-5 (FATP5) deficiency enhances hepatocellular carcinoma progression and metastasis by reprogramming cellular energy metabolism and regulating the AMPK-mTOR signaling pathway. Oncogenesis. 2021;10:74. https://doi.org/10.1038/s41389-021-00364-5.
Chu Z, Huo N, Zhu X, Liu H, Cong R, Ma L, et al. FOXO3A-induced LINC00926 suppresses breast tumor growth and metastasis through inhibition of PGK1-mediated Warburg effect. Mol Ther. 2021;29:2737–53. https://doi.org/10.1016/j.ymthe.2021.04.036.
Dai W, Xiang W, Han L, Yuan Z, Wang R, Ma Y, et al. PTPRO represses colorectal cancer tumorigenesis and progression by reprogramming fatty acid metabolism. Cancer Commun. 2022;42:848–67. https://doi.org/10.1002/cac2.12341.
Rozeveld CN, Johnson KM, Zhang L, Razidlo GL. KRAS Controls Pancreatic Cancer Cell Lipid Metabolism and Invasive Potential through the Lipase HSL. Cancer Res. 2020;80:4932–45. https://doi.org/10.1158/0008-5472.CAN-20-1255.
Wang Y, Chen J, Lu J, Xi J, Xu Z, Fan L, et al. Metal ions/nucleotide coordinated nanoparticles comprehensively suppress tumor by synergizing ferroptosis with energy metabolism interference. J Nanobiotechnol. 2022;20:199. https://doi.org/10.1186/s12951-022-01405-w.
Meng WR, Hao YY, He CS, Li L, Zhu G. Exosome-orchestrated hypoxic tumor microenvironment. Mol Cancer. 2019;18:57. https://doi.org/10.1186/s12943-019-0982-6.
Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016;352:175–80.
Godet I, Shin YJ, Ju JA, Ye IC, Wang G, Gilkes DM. Fate-mapping post-hypoxic tumor cells reveals a ROS-resistant phenotype that promotes metastasis. Nat Commun. 2019;10:4862. https://doi.org/10.1038/s41467-019-12412-1.
Wels J, Kaplan RN, Rafii S, Lyden D. Migratory neighbors and distant invaders: tumor-associated niche cells. Genes Dev. 2008;22:559–74. https://doi.org/10.1101/gad.1636908.
Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–34. https://doi.org/10.1182/blood-2004-03-1109.
Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. https://doi.org/10.1038/nrc2618.
Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430–9. https://doi.org/10.1038/nrc3726.
Gwak H, Park S, Kim J, Lee JD, Kim IS, Kim SI, et al. Microfluidic chip for rapid and selective isolation of tumor-derived extracellular vesicles for early diagnosis and metastatic risk evaluation of breast cancer. Biosens Bioelectron. 2021;192:113495. https://doi.org/10.1016/j.bios.2021.113495.
Liu W, Song J, Du X, Zhou Y, Li Y, Li R, et al. AKR1B10 (Aldo-keto reductase family 1 B10) promotes brain metastasis of lung cancer cells in a multi-organ microfluidic chip model. Acta Biomater. 2019;91:195–208. https://doi.org/10.1016/j.actbio.2019.04.053.
Yoshida GJ. Applications of patient-derived tumor xenograft models and tumor organoids. J Hematol Oncol. 2020;13:4. https://doi.org/10.1186/s13045-019-0829-z.
Du J, Lan T, Liao H, Feng X, Chen X, Liao W, et al. CircNFIB inhibits tumor growth and metastasis through suppressing MEK1/ERK signaling in intrahepatic cholangiocarcinoma. Mol Cancer. 2022;21:18. https://doi.org/10.1186/s12943-021-01482-9.
Bogani G, Mariani A, Paolini B, Ditto A, Raspagliesi F. Low-volume disease in endometrial cancer: The role of micrometastasis and isolated tumor cells. Gynecol Oncol. 2019;153:670–5. https://doi.org/10.1016/j.ygyno.2019.02.027.
Rampaul RS, Miremadi A, Pinder SE, A L, IO E. Pathological validation and significance of micrometastasis in sentinel nodes in primary breast cancer. Breast Cancer Res. 2001;3:113–6.
Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–9.
Sabouni E, Nejad MM, Mojtabavi S, Khoshduz S, Mojtabavi M, Nadafzadeh N, et al. Unraveling the function of epithelial-mesenchymal transition (EMT) in colorectal cancer: Metastasis, therapy response, and revisiting molecular pathways. Biomed Pharmacother. 2023;160:114395. https://doi.org/10.1016/j.biopha.2023.114395.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70.
Lv X, Lian Y, Liu Z, Xiao J, Zhang D, Yin X. Exosomal long non-coding RNA LINC00662 promotes non-small cell lung cancer progression by miR-320d/E2F1 axis. Aging. 2021;13:6010–24.
Zang XY, Gu JM, Zhang JY, Shi H, Hou SN, Xu X, et al. Exosome-transmitted lncRNA UFC1 promotes non-small-cell lung cancer progression by EZH2-mediated epigenetic silencing of PTEN expression. Cell Death Dis. 2020;11:215. https://doi.org/10.1038/s41419-020-2409-0.
Wu D, Deng S, Li L, Liu T, Zhang T, Li J, et al. TGF-beta1-mediated exosomal lnc-MMP2-2 increases blood-brain barrier permeability via the miRNA-1207-5p/EPB41L5 axis to promote non-small cell lung cancer brain metastasis. Cell Death Dis. 2021;12:721. https://doi.org/10.1038/s41419-021-04004-z.
Luan YP, Li X, Luan YQ, Zhao R, Li YM, Liu L, et al. Circulating lncRNA UCA1 Promotes Malignancy of Colorectal Cancer via the miR-143/MYO6 Axis. Mol Ther Nucleic Acids. 2020;19:790–803. https://doi.org/10.1016/j.omtn.2019.12.009.
Xu J, Xiao Y, Liu B, Pan S, Liu Q, Shan Y, et al. Exosomal MALAT1 sponges miR-26a/26b to promote the invasion and metastasis of colorectal cancer via FUT4 enhanced fucosylation and PI3K/Akt pathway. J Exp Clin Cancer Res. 2020;39:54. https://doi.org/10.1186/s13046-020-01562-6.
Liang ZX, Liu HS, Wang FW, Xiong L, Zhou C, Hu T, et al. LncRNA RPPH1 promotes colorectal cancer metastasis by interacting with TUBB3 and by promoting exosomes-mediated macrophage M2 polarization. Cell Death Dis. 2019;10:829. https://doi.org/10.1038/s41419-019-2077-0.
Dai W, Jin X, Han L, Huang H, Ji Z, Xu X, et al. Exosomal lncRNA DOCK9-AS2 derived from cancer stem cell-like cells activated Wnt/beta-catenin pathway to aggravate stemness, proliferation, migration, and invasion in papillary thyroid carcinoma. Cell Death Dis. 2020;11:743. https://doi.org/10.1038/s41419-020-02827-w.
Hardin H, Helein H, Meyer K, Robertson S, Zhang R, Zhong W, et al. Thyroid cancer stem-like cell exosomes: regulation of EMT via transfer of lncRNAs. Lab Invest. 2018;98:1133–42. https://doi.org/10.1038/s41374-018-0065-0.
Wu Q, He Y, Liu X, Luo F, Jiang Y, Xiang M, et al. Cancer stem cell-like cells-derived exosomal lncRNA CDKN2B-AS1 promotes biological characteristics in thyroid cancer via miR-122-5p/P4HA1 axis. Regen Ther. 2023;22:19–29. https://doi.org/10.1016/j.reth.2022.11.005.
Piao HY, Guo S, Wang Y, Zhang J. Exosome-transmitted lncRNA PCGEM1 promotes invasive and metastasis in gastric cancer by maintaining the stability of SNAI1. Clin Transl Oncol. 2021;23:246–56. https://doi.org/10.1007/s12094-020-02412-9.
Wang LY, Bo XT, Yi XY, Xiao XH, Zheng QH, Ma L, et al. Exosome-transferred LINC01559 promotes the progression of gastric cancer via PI3K/AKT signaling pathway. Cell Death Dis. 2020;11:723. https://doi.org/10.1038/s41419-020-02810-5.
Zhang C, Wang H, Liu Q, Dai S, Tian G, Wei X, et al. LncRNA CCAT1 facilitates the progression of gastric cancer via PTBP1-mediated glycolysis enhancement. J Exp Clin Cancer Res. 2023;42:246. https://doi.org/10.1186/s13046-023-02827-6.
Weigelt B, Peterse JL, van ‘t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. https://doi.org/10.1038/nrc1670.
Lu YH, Chen L, Li LD, Cao YQ. Exosomes Derived from Brain Metastatic Breast Cancer Cells Destroy the Blood-Brain Barrier by Carrying lncRNA GS1-600G8.5. Biomed Res Int. 2020;2020:7461727. https://doi.org/10.1155/2020/7461727.
Kong X, Li J, Li Y, Duan W, Qi Q, Wang T, et al. A novel long non-coding RNA AC073352.1 promotes metastasis and angiogenesis via interacting with YBX1 in breast cancer. Cell Death Dis. 2021;12:670. https://doi.org/10.1038/s41419-021-03943-x.
Farhadi S, Mohammadi-Yeganeh S, Kiani J, Hashemi SM, Koochaki A, Sharifi K, et al. Exosomal delivery of 7SK long non-coding RNA suppresses viability, proliferation, aggressiveness and tumorigenicity in triple negative breast cancer cells. Life Sci. 2023;322:121646. https://doi.org/10.1016/j.lfs.2023.121646.
Zheng H, Chen C, Luo Y, Yu M, He W, An M, et al. Tumor-derived exosomal BCYRN1 activates WNT5A/VEGF-C/VEGFR3 feedforward loop to drive lymphatic metastasis of bladder cancer. Clin Transl Med. 2021;11:e497. https://doi.org/10.1002/ctm2.497.
Huang CS, Ho JY, Chiang JH, Yu CP, Yu DS. Exosome-Derived LINC00960 and LINC02470 Promote the Epithelial-Mesenchymal Transition and Aggressiveness of Bladder Cancer Cells. Cells. 2020;9:1419. https://doi.org/10.3390/cells9061419.
Yan L, Wang PY, Fang WH, Liang CZ. Cancer-associated fibroblasts-derived exosomes-mediated transfer of LINC00355 regulates bladder cancer cell proliferation and invasion. Cell Biochem Funct. 2020;38:257–65. https://doi.org/10.1002/cbf.3462.
Lai YJ, Dong LH, Jin HF, Li HJ, Sun ML, Li JL. Exosome long non-coding RNA SOX2-OT contributes to ovarian cancer malignant progression by miR-181b-5p/SCD1 signaling. Aging. 2021;13:23726–38.
Jin N, Jin NQ, Bu WH, Li X, Liu L, Wang Z, et al. Long non-coding RNA TIRY promotes tumor metastasis by enhancing epithelial-to-mesenchymal transition in oral cancer. Exp Biol Med. 2020;245:585–96. https://doi.org/10.1177/1535370220903673.
Li ZR, Qin XB, Bian W, Li Y, Shan B, Yao Z, et al. Exosomal lncRNA ZFAS1 regulates esophageal squamous cell carcinoma cell proliferation, invasion, migration and apoptosis via microRNA-124/STAT3 axis. J Exp Clin Cancer Res. 2019;38:477–90. https://doi.org/10.1186/s13046-019-1473-8.
Takahashi K, Ota Y, Kogure T, Suzuki Y, Iwamoto H, Yamakita K, et al. Circulating extracellular vesicle-encapsulated HULC is a potential biomarker for human pancreatic cancer. Cancer Sci. 2020;111:98–111. https://doi.org/10.1111/cas.14232.
Wang XJ, Li HZ, Lu XX, Wen C, Huo Z, Shi M, et al. Melittin-induced long non-coding RNA NONHSAT105177 inhibits proliferation and migration of pancreatic ductal adenocarcinoma. Cell Death Dis. 2018;9:940. https://doi.org/10.1038/s41419-018-0965-3.
Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. https://doi.org/10.1038/ni1013.
Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5:145. https://doi.org/10.1038/s41392-020-00261-0.
Chen CH, Luo YM, He W, Zhao Y, Kong Y, Liu H, et al. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J Clin Invest. 2020;130:404–21. https://doi.org/10.1172/JCI130892.
You LN, Tai QW, Xu L, Hao Y, Guo WJ, Zhang Q, et al. Exosomal LINC00161 promotes angiogenesis and metastasis via regulating miR-590-3p/ROCK axis in hepatocellular carcinoma. Cancer Gene Ther. 2021;28:719–36. https://doi.org/10.1038/s41417-020-00269-2.
Guo Z, Wang X, Yang Y, Chen W, Zhang K, Teng B, et al. Hypoxic Tumor-Derived Exosomal Long Noncoding RNA UCA1 Promotes Angiogenesis via miR-96-5p/AMOTL2 in Pancreatic Cancer. Mol Ther Nucleic Acids. 2020;22:179–95. https://doi.org/10.1016/j.omtn.2020.08.021.
Zhang C, Luo Y, Cao J, Wang X, Miao Z, Shao G. Exosomal lncRNA FAM225A accelerates esophageal squamous cell carcinoma progression and angiogenesis via sponging miR-206 to upregulate NETO2 and FOXP1 expression. Cancer Med. 2020;9:8600–11. https://doi.org/10.1002/cam4.3463.
Su CY, Zhang JY, Yarden Y, Fu LW. The key roles of cancer stem cell-derived extracellular vesicles. Signal Transduct Target Ther. 2021;6:109. https://doi.org/10.1038/s41392-021-00499-2.
Jiang XC, Yan YK, Hu MH, Chen X, Wang Y, Dai Y, et al. Increased level of H19 long noncoding RNA promotes invasion, angiogenesis, and stemness of glioblastoma cells. J Neurosurg. 2016;124:129–36. https://doi.org/10.3171/2014.12.JNS1426.
Shima H, Kida K, Adachi S, Yamada A, Sugae S, Narui K, et al. Lnc RNA H19 is associated with poor prognosis in breast cancer patients and promotes cancer stemness. Breast Cancer Res Treat. 2018;170:507–16. https://doi.org/10.1007/s10549-018-4793-z.
Ye J, Wang Z, Zhao J, Chen W, Wu D, Wu P, et al. MicroRNA-141 inhibits tumor growth and minimizes therapy resistance in colorectal cancer. Mol Med Rep. 2017;15:1037–42. https://doi.org/10.3892/mmr.2017.6135.
Ren J, Ding L, Zhang DY, Shi G, Xu Q, Shen S, et al. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics. 2018;8:3932–48. https://doi.org/10.7150/thno.25541.
Domvri K, Petanidis S, Anestakis D, Porpodis K. Exosomal lncRNA PCAT-1 promotes Kras-associated chemoresistance via immunosuppressive miR-182/miR-217 signaling and p27/CDK6 regulation. Oncotarget. 2020;11:2847–62.
Li W, Zhang LY, Guo BB, Deng J, Wu S, Li F, et al. Exosomal FMR1-AS1 facilitates maintaining cancer stem-like cell dynamic equilibrium via TLR7/NFkappaB/c-Myc signaling in female esophageal carcinoma. Mol Cancer. 2019;18:22. https://doi.org/10.1186/s12943-019-0949-7.
Ni C, Fang QQ, Chen WZ, Jiang JX, Jiang Z, Ye J, et al. Breast cancer-derived exosomes transmit lncRNA SNHG16 to induce CD73+gammadelta1 Treg cells. Signal Transduct Target Ther. 2020;5:41. https://doi.org/10.1038/s41392-020-0129-7.
Fan F, Chen KJ, Lu XL, Li AJ, Liu C, Wu B. Dual targeting of PD-L1 and PD-L2 by PCED1B-AS1 via sponging hsa-miR-194-5p induces immunosuppression in hepatocellular carcinoma. Hepatol Int. 2020;15:444–58. https://doi.org/10.1007/s12072-020-10101-6.
Xie Z, Xia J, Jiao M, Zhao P, Wang Z, Lin S, et al. Exosomal lncRNA HOTAIR induces PDL1(+) B cells to impede anti-tumor immunity in colorectal cancer. Biochem Biophys Res Commun. 2023;644:112–21. https://doi.org/10.1016/j.bbrc.2023.01.005.
Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. https://doi.org/10.1016/j.immuni.2014.06.010.
Kerkar SP, Restifo NP. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res. 2012;72:3125–30. https://doi.org/10.1158/0008-5472.CAN-11-4094.
Chen J, Zhang K, Zhi Y, Wu Y, Chen B, Bai J, et al. Tumor-derived exosomal miR-19b-3p facilitates M2 macrophage polarization and exosomal LINC00273 secretion to promote lung adenocarcinoma metastasis via Hippo pathway. Clin Transl Med. 2021;11:e478. https://doi.org/10.1002/ctm2.478.
Liang YR, Song XJ, Li YM, Chen B, Zhao W, Wang L, et al. LncRNA BCRT1 promotes breast cancer progression by targeting miR-1303/PTBP3 axis. Mol Cancer. 2020;19:85. https://doi.org/10.1186/s12943-020-01206-5.
Ji W, Bai J, Ke Y. Exosomal ZFPM2-AS1 contributes to tumorigenesis, metastasis, stemness, macrophage polarization, and infiltration in hepatocellular carcinoma through PKM mediated glycolysis. Environ Toxicol. 2023;38:1332–46. https://doi.org/10.1002/tox.23767.
Wang JS, Yang K, Yuan WX, Gao ZY. Determination of Serum Exosomal H19 as a Noninvasive Biomarker for Bladder Cancer Diagnosis and Prognosis. Med Sci Monit. 2018;24:9307–16. https://doi.org/10.12659/MSM.912018.
Lei Y, Guo W, Chen BW, Chen L, Gong J, Li W. Tumorreleased lncRNA H19 promotes gefitinib resistance via packaging into exosomes in nonsmall cell lung cancer. Oncol Rep. 2018;40:3438–46. https://doi.org/10.3892/or.2018.6762.
Zhang Y, Liu YT, Tang H, Xie WQ, Yao H, Gu WT, et al. Exosome-Transmitted lncRNA H19 Inhibits the Growth of Pituitary Adenoma. J Clin Endocrinol Metab. 2019;104:6345–56. https://doi.org/10.1210/jc.2019-00536.
Chen LS, Yao HB, Wang K, Liu XF. Long Non-Coding RNA MALAT1 Regulates ZEB1 Expression by Sponging miR-143-3p and Promotes Hepatocellular Carcinoma Progression. J Cell Biochem. 2017;118:4836–43. https://doi.org/10.1002/jcb.26158.
Hsu XR, Wu JE, Wu YY, Hsiao SY, Liang JL, Wu YJ, et al. Exosomal long noncoding RNA MLETA1 promotes tumor progression and metastasis by regulating the miR-186-5p/EGFR and miR-497-5p/IGF1R axes in non-small cell lung cancer. J Exp Clin Cancer Res. 2023;42:283. https://doi.org/10.1186/s13046-023-02859-y.
Zhang P, Zhou HX, Lu KF, Lu YO, Wang Y, Feng T. Exosome-mediated delivery of MALAT1 induces cell proliferation in breast cancer. Onco Targets Ther. 2018;11:291–9. https://doi.org/10.2147/OTT.S155134.
Qiu JJ, Lin XJ, Tang XY, Zheng TT, Lin YY, Hua KQ. Exosomal Metastasis‑Associated Lung Adenocarcinoma Transcript 1 Promotes Angiogenesis and Predicts Poor Prognosis in Epithelial Ovarian Cancer. Int J Biol Sci. 2018;14:1960–73. https://doi.org/10.7150/ijbs.28048.
Chen P, Liu Z, Xiao H, Yang X, Li T, Huang W, et al. Effect of tumor exosome-derived Lnc RNA HOTAIR on the growth and metastasis of gastric cancer. Clin Transl Oncol. 2023;25:3447–59. https://doi.org/10.1007/s12094-023-03208-3.
Berrondo C, Flax J, Kucherov V, Siebert A, Osinski T, Rosenberg A, et al. Expression of the Long Non-Coding RNA HOTAIR Correlates with Disease Progression in Bladder Cancer and Is Contained in Bladder Cancer Patient Urinary Exosomes. PLoS One. 2016;11:e0147236. https://doi.org/10.1371/journal.pone.0147236.
Zhang CC, Xu LS, Deng G, Ding Y, Bi K, Jin H, et al. Exosomal HOTAIR promotes proliferation, migration and invasion of lung cancer by sponging miR-203. Sci China Life Sci. 2020;63:1265–8. https://doi.org/10.1007/s11427-019-1579-x.
Wang YL, Liu LC, Hung Y, Chen CJ, Lin YZ, Wu WR, et al. Long non-coding RNA HOTAIR in circulatory exosomes is correlated with ErbB2/HER2 positivity in breast cancer. Breast. 2019;46:64–9. https://doi.org/10.1016/j.breast.2019.05.003.
Tan SK, Pastori C, Penas C, Komotar RJ, Ivan ME, Wahlestedt C, et al. Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol Cancer. 2018;17:74. https://doi.org/10.1186/s12943-018-0822-0.
Zhang Y, Li M, Gao X, Chen Y, Liu T. Nanotechnology in cancer diagnosis: progress, challenges and opportunities. J Hematol Oncol. 2019;12:137. https://doi.org/10.1186/s13045-019-0833-3.
Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14:85. https://doi.org/10.1186/s13045-021-01096-0.
Kok VC, Yu CC. Cancer-Derived Exosomes: Their Role in Cancer Biology and Biomarker Development. Int J Nanomed. 2020;15:8019–36. https://doi.org/10.2147/IJN.S272378.
Huang X, Wu W, Jing D, Yang L, Guo H, Wang L, et al. Engineered exosome as targeted lncRNA MEG3 delivery vehicles for osteosarcoma therapy. J Control Release. 2022;343:107–17. https://doi.org/10.1016/j.jconrel.2022.01.026.
Nie W, Wu G, Zhang J, Huang LL, Ding J, Jiang A, et al. Responsive Exosome Nano-bioconjugates for Synergistic Cancer Therapy. Angew Chem Int Ed Engl. 2020;59:2018–22. https://doi.org/10.1002/anie.201912524.
Li J, Li J, Peng Y, Du Y, Yang Z, Qi X. Dendritic cell derived exosomes loaded neoantigens for personalized cancer immunotherapies. J Control Rel. 2023;353:423–33. https://doi.org/10.1016/j.jconrel.2022.11.053.
Xu X, Liu C, Wang Y, Koivisto O, Zhou J, Shu Y, et al. Nanotechnology-based delivery of CRISPR/Cas9 for cancer treatment. Adv Drug Deliv Rev. 2021;176:113891. https://doi.org/10.1016/j.addr.2021.113891.
Chowdhry R, Lu SZ, Lee S, Godhulayyagari S, Ebrahimi SB, Samanta D. Enhancing CRISPR/Cas systems with nanotechnology. Trends Biotechnol. 2023;41:1549–64. https://doi.org/10.1016/j.tibtech.2023.06.005.
Ali HS, Boshra MS, El Meteini MS, Shafei AE, Matboli M. lncRNA- RP11-156p1.3, novel diagnostic and therapeutic targeting via CRISPR/Cas9 editing in hepatocellular carcinoma. Genomics. 2020;112:3306–14. https://doi.org/10.1016/j.ygeno.2020.06.020.
Raza A, Khan AQ, Inchakalody VP, Mestiri S, Yoosuf Z, Bedhiafi T, et al. Dynamic liquid biopsy components as predictive and prognostic biomarkers in colorectal cancer. J Exp Clin Cancer Res. 2022;41:99. https://doi.org/10.1186/s13046-022-02318-0.
Guo T, Tang XH, Gao XY, Zhou Y, Jin B, Deng ZQ, et al. A liquid biopsy signature of circulating exosome-derived mRNAs, miRNAs and lncRNAs predict therapeutic efficacy to neoadjuvant chemotherapy in patients with advanced gastric cancer. Mol Cancer. 2022;21:216. https://doi.org/10.1186/s12943-022-01684-9.
De Los Santos MC, Dragomir MP, Calin GA. The role of exosomal long non-coding RNAs in cancer drug resistance. Cancer Drug Resist. 2019;2:1178–92. https://doi.org/10.20517/cdr.2019.74.
Qu L, Ding J, Chen C, Wu ZJ, Liu B, Gao Y, et al. Exosome-Transmitted lncARSR Promotes Sunitinib Resistance in Renal Cancer by Acting as a Competing Endogenous RNA. Cancer Cell. 2016;29:653–68. https://doi.org/10.1016/j.ccell.2016.03.004.
Yu Z, Tang H, Chen S, Xie Y, Shi L, Xia S, et al. Exosomal LOC85009 inhibits docetaxel resistance in lung adenocarcinoma through regulating ATG5-induced autophagy. Drug Resist Updat. 2023;67:100915. https://doi.org/10.1016/j.drup.2022.100915.
Liu X, Zhang G, Yu T, Liu J, Chai X, Yin D, et al. CL4-modified exosomes deliver lncRNA DARS-AS1 siRNA to suppress triple-negative breast cancer progression and attenuate doxorubicin resistance by inhibiting autophagy. Int J Biol Macromol. 2023;250:126147. https://doi.org/10.1016/j.ijbiomac.2023.126147.
Yi K, Wang Y, Rong Y, Bao Y, Liang Y, Chen Y, et al. Transcriptomic Signature of 3D Hierarchical Porous Chip Enriched Exosomes for Early Detection and Progression Monitoring of Hepatocellular Carcinoma. Adv Sci (Weinh). 2024;11:e2305204. https://doi.org/10.1002/advs.202305204.
Guo C, Ma X, Gao F, Guo Y. Off-target effects in CRISPR/Cas9 gene editing. Front Bioeng Biotechnol. 2023;11:1143157. https://doi.org/10.3389/fbioe.2023.1143157.
Cheng X, Li Z, Shan R, Li Z, Wang S, Zhao W, et al. Modeling CRISPR-Cas13d on-target and off-target effects using machine learning approaches. Nat Commun. 2023;14:752. https://doi.org/10.1038/s41467-023-36316-3.
Xia Y, Zhang J, Liu G, Wolfram J. Immunogenicity of Extracellular Vesicles. Adv Mater. 2024:e2403199. https://doi.org/10.1002/adma.202403199.
Wang F, Fu K, Wang Y, Pan C, Wang X, Liu Z, et al. Small-molecule agents for cancer immunotherapy. Acta Pharm Sin B. 2024;14:905–52. https://doi.org/10.1016/j.apsb.2023.12.010.
Lin LY, Yang L, Zeng Q, Wang L, Chen ML, Zhao ZH, et al. Tumor-originated exosomal lncUEGC1 as a circulating biomarker for early-stage gastric cancer. Mol Cancer. 2018;17:84. https://doi.org/10.1186/s12943-018-0834-9.
Zhao R, Zhang YL, Zhang X, Yang Y, Zheng X, Li X, et al. Exosomal long noncoding RNA HOTTIP as potential novel diagnostic and prognostic biomarker test for gastric cancer. Mol Cancer. 2018;17:68. https://doi.org/10.1186/s12943-018-0817-x.
Pan L, Liang W, Fu M, Huang ZH, Li X, Zhang W, et al. Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J Cancer Res Clin Oncol. 2017;143:991–1004. https://doi.org/10.1007/s00432-017-2361-2.
Guo X, Lv X, Ru Y, Zhou F, Wang N, Xi H, et al. Circulating Exosomal Gastric Cancer-Associated Long Noncoding RNA1 as a Biomarker for Early Detection and Monitoring Progression of Gastric Cancer: A Multiphase Study. JAMA Surg. 2020;155:572–9. https://doi.org/10.1001/jamasurg.2020.1133.
Zhao W, Zhang Y, Zhang W, Sun Y, Zheng B, Wang J, et al. Exosomal LINC00355 promotes the malignant progression of gastric cancer through histone deacetylase HDAC3-mediated TP53INP1 transcriptional inhibition. Life Sci. 2023;315:121387. https://doi.org/10.1016/j.lfs.2023.121387.
Ma B, Wang J, Yusufu P. Tumor‐derived exosome ElNF1‐AS1 affects the progression of gastric cancer by promoting M2 polarization of macrophages. Environ Toxicol. 2023;38:2228–39. https://doi.org/10.1002/tox.23862.
Liu T, Zhang X, Gao SY, Jing F. Exosomal long noncoding RNA CRNDE-h as a novel serum-based biomarker for diagnosis and prognosis of colorectal cancer. Oncotarget. 2016;7:85551–63.
Dong L, Lin W, Qi P, Xu MD, Wu X, Ni S, et al. Circulating Long RNAs in Serum Extracellular Vesicles: Their Characterization and Potential Application as Biomarkers for Diagnosis of Colorectal Cancer. Cancer Epidemiol Biomark Prev. 2016;25:1158–66. https://doi.org/10.1158/1055-9965.EPI-16-0006.
Zhao WM, Song M, Zhang J, Kuerban M, Wang HJ. Combined identification of long non-coding RNA CCAT1 and HOTAIR in serum as an effective screening for colorectal carcinoma. Int J Clin Exp Pathol. 2015;8:14131–40.
Zhao YH, Du TT, Du LT, Li P, Li J, Duan W, et al. Long noncoding RNA LINC02418 regulates MELK expression by acting as a ceRNA and may serve as a diagnostic marker for colorectal cancer. Cell Death Dis. 2019;10:568. https://doi.org/10.1038/s41419-019-1804-x.
Zheng R, Du ML, Wang XW, Xu W, Liang J, Wang W, et al. Exosome-transmitted long non-coding RNA PTENP1 suppresses bladder cancer progression. Mol Cancer. 2018;17:143. https://doi.org/10.1186/s12943-018-0880-3.
Zhan Y, Du LT, Wang LS, Jiang X, Zhang S, Li J, et al. Expression signatures of exosomal long non-coding RNAs in urine serve as novel non-invasive biomarkers for diagnosis and recurrence prediction of bladder cancer. Mol Cancer. 2018;17:142. https://doi.org/10.1186/s12943-018-0893-y.
Xue M, Chen W, Xiang A, Wang R, Chen H, Pan J, et al. Hypoxic exosomes facilitate bladder tumor growth and development through transferring long non-coding RNA-UCA1. Mol Cancer. 2017;16:143. https://doi.org/10.1186/s12943-017-0714-8.
Zheng R, Gao F, Mao Z, Xiao Y, Yuan L, Huang Z, et al. LncRNA BCCE4 Genetically Enhances the PD-L1/PD-1 Interaction in Smoking-Related Bladder Cancer by Modulating miR-328-3p-USP18 Signaling. Adv Sci. 2023;10:e2303473. https://doi.org/10.1002/advs.202303473.
Li BG, Mao R, Liu CF, Zhang W, Tang Y, Guo Z. LncRNA FAL1 promotes cell proliferation and migration by acting as a CeRNA of miR-1236 in hepatocellular carcinoma cells. Life Sci. 2018;197:122–9. https://doi.org/10.1016/j.lfs.2018.02.006.
Ni Q, Zhang H, Shi X, Li X. Exosomal lncRNA HCG18 contributes to cholangiocarcinoma growth and metastasis through mediating miR-424-5p/SOX9 axis through PI3K/AKT pathway. Cancer Gene Ther. 2023;30:582–95. https://doi.org/10.1038/s41417-022-00500-2.
Zhang J, Cai M, Jiang DW, Xu L. Upregulated LncRNA-CCAT1 promotes hepatocellular carcinoma progression by functioning as miR-30c-2-3p sponge. Cell Biochem Funct. 2019;37:84–92. https://doi.org/10.1002/cbf.3375.
Sun L, Su YY, Liu XX, Xu M, Chen X, Zhu Y, et al. Serum and exosome long non coding RNAs as potential biomarkers for hepatocellular carcinoma. J Cancer. 2018;9:2631–9. https://doi.org/10.7150/jca.24978.
Huang XJ, Sun LY, Wen S, Deng D, Wan F, He X, et al. RNA sequencing of plasma exosomes revealed novel functional long noncoding RNAs in hepatocellular carcinoma. Cancer Sci. 2020;111:3338–49. https://doi.org/10.1111/cas.14516.
Yao J, Hua X, Shi J, Hu X, Lui K, He K, et al. LncRNA THEMIS2-211, a tumor-originated circulating exosomal biomarker, promotes the growth and metastasis of hepatocellular carcinoma by functioning as a competing endogenous RNA. FASEB J. 2022;36:e22238. https://doi.org/10.1096/fj.202101564R.
Teng Y, Kang H, Chu Y. Identification of an Exosomal Long Noncoding RNA SOX2-OT in Plasma as a Promising Biomarker for Lung Squamous Cell Carcinoma. Genet Test Mol Biomark. 2019;23:235–40. https://doi.org/10.1089/gtmb.2018.0103.
Cheng Y, Dai X, Yang T, Zhang N, Liu Z, Jiang Y. Low Long Noncoding RNA Growth Arrest-Specific Transcript 5 Expression in the Exosomes of Lung Cancer Cells Promotes Tumor Angiogenesis. J Oncol. 2019;2019:2476175. https://doi.org/10.1155/2019/2476175.
Zhang XL, Guo HH, Bao Y, Yu HM, Xie D, Wang X. Exosomal long non‑coding RNA DLX6‑AS1 as a potential diagnostic biomarker for non‑small cell lung cancer. Oncol Lett. 2019;18:5197–204. https://doi.org/10.3892/ol.2019.10892.
Zhang R, Xia YH, Wang ZX, Zheng J, Chen Y, Li X, et al. Serum long non coding RNA MALAT-1 protected by exosomes is up-regulated and promotes cell proliferation and migration in non-small cell lung cancer. Biochem Biophys Res Commun. 2017;490:406–14. https://doi.org/10.1016/j.bbrc.2017.06.055.
Min L, Zhu T, Lv B, An T, Zhang Q, Shang Y, et al. Exosomal LncRNA RP5-977B1 as a novel minimally invasive biomarker for diagnosis and prognosis in non-small cell lung cancer. Int J Clin Oncol. 2022;27:1013–24. https://doi.org/10.1007/s10147-022-02129-5.
Cheng C, Zhang ZC, Cheng FL, Shao ZW. Exosomal lncRNA RAMP2-AS1 Derived from Chondrosarcoma Cells Promotes Angiogenesis Through miR-2355-5p/VEGFR2 Axis. Onco Targets Ther. 2020;13:3291–301. https://doi.org/10.2147/OTT.S244652.
Wang B, Wang X, Li P, Niu X, Liang X, Liu G, et al. Osteosarcoma Cell-Derived Exosomal ELFN1-AS1 Mediates Macrophage M2 Polarization via Sponging miR-138-5p and miR-1291 to Promote the Tumorgenesis of Osteosarcoma. Front Oncol. 2022;12:881022. https://doi.org/10.3389/fonc.2022.881022.
Chang X, Tan Q, Xu J, Wu X, Wang Y, Zhang Y, et al. Tumor-derived exosomal linc00881 induces lung fibroblast activation and promotes osteosarcoma lung migration. Cancer Cell Int. 2023;23. https://doi.org/10.1186/s12935-023-03121-3.
Li QM, Wang XD, Jiang N, Xie X, Liu N, Liu J, et al. Exosome-transmitted linc00852 associated with receptor tyrosine kinase AXL dysregulates the proliferation and invasion of osteosarcoma. Cancer Med. 2020;9:6354–66. https://doi.org/10.1002/cam4.3303.
Zhao W, Qin P, Zhang D, Cui XC, Guo J, Yu ZZ. Long non-coding RNA PVT1 encapsulated in bone marrow mesenchymal stem cell-derived exosomes promotes osteosarcoma growth and metastasis by stabilizing ERG and sponging miR-183-5p. Aging. 2019;11:9581–96.
Zhong GB, Wang KQ, Li JW, Xiao S, Wei W, Liu J. Determination of Serum Exosomal H19 as a Noninvasive Biomarker for Breast Cancer Diagnosis. Onco Targets Ther. 2020;13:2563–71. https://doi.org/10.2147/OTT.S243601.
He Z, Wang J, Zhu C, Xu J, Chen P, Jiang X, et al. Exosome-derived FGD5-AS1 promotes tumor-associated macrophage M2 polarization-mediated pancreatic cancer cell proliferation and metastasis. Cancer Lett. 2022;548:215751. https://doi.org/10.1016/j.canlet.2022.215751.
Sun Z, Sun D, Feng Y, Zhang B, Sun P, Zhou B, et al. Exosomal linc-ROR mediates crosstalk between cancer cells and adipocytes to promote tumor growth in pancreatic cancer. Mol Ther Nucleic Acids. 2021;26:253–68. https://doi.org/10.1016/j.omtn.2021.06.001.
Feng Z, Li K, Qin K, Liang J, Shi M, Ma Y, et al. The LINC00623/NAT10 signaling axis promotes pancreatic cancer progression by remodeling ac4C modification of mRNA. J Hematol Oncol. 2022;15:112. https://doi.org/10.1186/s13045-022-01338-9.
Li ZH, Jiang P, Li J, Peng M, Zhao X, Zhang X, et al. Tumor-derived exosomal lnc-Sox2ot promotes EMT and stemness by acting as a ceRNA in pancreatic ductal adenocarcinoma. Oncogene. 2018;37:3822–38. https://doi.org/10.1038/s41388-018-0237-9.
Huang LJ, Wang Y, Chen J, Wang Y, Zhao Y, Wang Y, et al. Long noncoding RNA PCAT1, a novel serum-based biomarker, enhances cell growth by sponging miR-326 in oesophageal squamous cell carcinoma. Cell Death Dis. 2019;10:513. https://doi.org/10.1038/s41419-019-1745-4.
Liu J, Zhou WY, Luo XJ, Chen YX, Wong CW, Liu ZX, et al. Long noncoding RNA Regulating ImMune Escape regulates mixed lineage leukaemia protein-1-H3K4me3-mediated immune escape in oesophageal squamous cell carcinoma. Clin Transl Med. 2023;13:e1410. https://doi.org/10.1002/ctm2.1410.
Jiao ZC, Yu A, Rong WW, He XF. Five-lncRNA signature in plasma exosomes serves as diagnostic biomarker for esophageal squamous cell carcinoma. Aging. 2020;12:18632–103559.
Zhang ZR, Yin JX, Lu CF, Wei Y, Zeng A, You Y. Exosomal transfer of long non-coding RNA SBF2-AS1 enhances chemoresistance to temozolomide in glioblastoma. J Exp Clin Cancer Res. 2019;38:166. https://doi.org/10.1186/s13046-019-1139-6.
Li J, Liao T, Liu H, Yuan H, Ouyang T, Wang J, et al. Hypoxic Glioma Stem Cell-Derived Exosomes Containing Linc01060 Promote Progression of Glioma by Regulating the MZF1/c-Myc/HIF1alpha Axis. Cancer Res. 2021;81:114–28. https://doi.org/10.1158/0008-5472.CAN-20-2270.
Huang XX, Liu XM, Du B, Liu XL, Xue M, Yan Q, et al. LncRNA LINC01305 promotes cervical cancer progression through KHSRP and exosome-mediated transfer. Aging. 2021;13:19230–42.
Gao Z, Wang Q, Ji M, Guo X, Li L, Su X. Exosomal lncRNA UCA1 modulates cervical cancer stem cell self-renewal and differentiation through microRNA-122-5p/SOX2 axis. J Transl Med. 2021;19:229. https://doi.org/10.1186/s12967-021-02872-9.
Funding
This work was supported by the National Key R&D Program of China (2021YFE0202000), China-Bulgaria Science and Technology Cooperation Committee 18th Regular Meeting Exchange Project (18-9), the Affiliated Qingyuan Hospital of Guangzhou Medical University (202301-301), The Affiliated Traditional Chinese Medicine Hospital, Guangzhou Medical University (20240068JJ), Guangzhou Medical University Undergraduates project (pdjh 2022b0425, 02-410-2206301).
Author information
Authors and Affiliations
Contributions
Zhile Yu, Jiali Fu and Yusong Wu: wrote the manuscript; Tungalag Battulga, Dianchang Wen: completed the figures and tables; Vanya Mantareva, Ivica Blažević: revised the manuscript; Yuqing Wang, Jianye Zhang: conceived and revised the manuscript; All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, Z., Fu, J., Mantareva, V. et al. The role of tumor-derived exosomal LncRNA in tumor metastasis. Cancer Gene Ther 32, 273–285 (2025). https://doi.org/10.1038/s41417-024-00852-x
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41417-024-00852-x