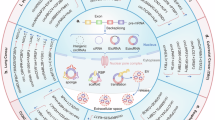
Abstract
Cancer remains a major threat to human health, with chemotherapy serving as one of the main treatment strategies to alleviate patient suffering. However, prolonged chemotherapy often leads to the development of drug resistance, complicating treatment outcomes. Cisplatin, a commonly utilized chemotherapeutic agent, demonstrates efficacy against a range of cancers but frequently encounters resistance, posing a significant challenge in tumor management and prognosis. Drug resistance not only facilitates tumor progression but also reduces survival rates, highlighting the urgent need for innovative strategies to overcome this issue. In recent years, non-coding RNAs, particularly circular RNAs (circRNAs), have gained attention in cancer therapy due to their stability and specificity. Moreover, an increasing number of studies have reported that circRNAs are involved in cisplatin resistance across various types of cancer. This paper primarily reviews the mechanisms and roles of circRNA in mediating cisplatin resistance over the past 3 years. These findings highlight circRNAs as promising therapeutic targets for overcoming cancer drug resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Hellmann MD, Li BT, Chaft JE, Kris MG. Chemotherapy remains an essential element of personalized care for persons with lung cancers. Ann Oncol. 2016;27:1829–35. https://doi.org/10.1093/annonc/mdw271.
Zhang Y, Liu Z, Zhong Z, Ji Y, Guo H, Wang W, et al. A tumor suppressor protein encoded by circKEAP1 inhibits osteosarcoma cell stemness and metastasis by promoting vimentin proteasome degradation and activating anti-tumor immunity. J Exp Clin Cancer Res. 2024;43:52. https://doi.org/10.1186/s13046-024-02971-7.
Zhang F, Wei D, Xie S, Ren L, Qiao S, Li L, et al. CircZCCHC2 decreases pirarubicin sensitivity and promotes triple-negative breast cancer development via the miR-1200/TPR axis. iScience. 2024;27:109057. https://doi.org/10.1016/j.isci.2024.109057.
Xu T, Xiong M, Hong Q, Pan B, Xu M, Wang Y, et al. Hsa_circ_0007990 promotes breast cancer growth via inhibiting YBX1 protein degradation to activate E2F1 transcription. Cell Death Dis. 2024;15:153. https://doi.org/10.1038/s41419-024-06527-7.
Makovec T. Cisplatin and beyond: molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol Oncol. 2019;53:148–58. https://doi.org/10.2478/raon-2019-0018.
Qu B, Liu J, Peng Z, Xiao Z, Li S, Wu J, et al. CircSOD2 polarizes macrophages towards the M1 phenotype to alleviate cisplatin resistance in gastric cancer cells by targeting the miR-1296/STAT1 axis. Gene. 2023;887:147733. https://doi.org/10.1016/j.gene.2023.147733.
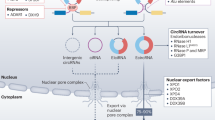
Chen LL, Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12:381–8. https://doi.org/10.1080/15476286.2015.1020271.
Li Z, Huang C, Bao C, Chen L, Lin M, Wang X, et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22:256–64. https://doi.org/10.1038/nsmb.2959.
Wang Y, Liu J, Ma J, Sun T, Zhou Q, Wang W, et al. Exosomal circRNAs: biogenesis, effect and application in human diseases. Mol Cancer. 2019;18:116. https://doi.org/10.1186/s12943-019-1041-z.
Misir S, Wu N, Yang BB. Specific expression and functions of circular RNAs. Cell Death Differ. 2022;29:481–91. https://doi.org/10.1038/s41418-022-00948-7.
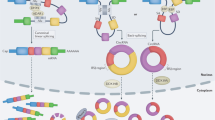
Bak RO, Mikkelsen JG. miRNA sponges: soaking up miRNAs for regulation of gene expression. Wiley Interdiscip Rev RNA. 2014;5:317–33. https://doi.org/10.1002/wrna.1213.
Liu Z, Zhou Y, Liang G, Ling Y, Tan W, Tan L, et al. Circular RNA hsa_circ_001783 regulates breast cancer progression via sponging miR-200c-3p. Cell Death Dis. 2019;10:55 https://doi.org/10.1038/s41419-018-1287-1.
Qiao L, Li CG, LiuD. CircRNA_0048211 protects postmenopausal osteoporosis through targeting miRNA-93-5p to regulate BMP2. Eur Rev Med Pharmacol Sci.2020;24:3459–66. 10.26355/eurrev_202004_20804
Zhao Z, Yang W, Kong R, Zhang Y, Li L, Song Z, et al. circEIF3I facilitates the recruitment of SMAD3 to early endosomes to promote TGF-beta signalling pathway-mediated activation of MMPs in pancreatic cancer. Mol Cancer. 2023;22:152.https://doi.org/10.1186/s12943-023-01847-2.
Zhang Y, Jiang J, Zhang J, Shen H, Wang M, Guo Z, et al. CircDIDO1 inhibits gastric cancer progression by encoding a novel DIDO1-529aa protein and regulating PRDX2 protein stability. Mol Cancer. 2021;20:101. https://doi.org/10.1186/s12943-021-01390-y.
Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78. https://doi.org/10.1016/j.ejphar.2014.07.025.
Rosenberg B, Vancamp L, Krigas T. Inhibition of cell division in Escherichia Coli by electrolysis products from a platinum electrode. Nature. 1965;205:698–9. https://doi.org/10.1038/205698a0.
Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–84. https://doi.org/10.1038/nrc2167.
Ranasinghe R, Mathai ML, Zulli A. Cisplatin for cancer therapy and overcoming chemoresistance. Heliyon. 2022;8:e10608. https://doi.org/10.1016/j.heliyon.2022.e10608.
MATSUMOTO K. Inorganic and Organometallic Chemistry of Cisplatin-Derived Diplatinum(III) Complexes [M]. Cisplatin. 1999;455–75. https://doi.org/10.1002/9783906390420.ch18.
Amable L. Cisplatin resistance and opportunities for precision medicine. Pharmacol Res. 2016;106:27–36. https://doi.org/10.1016/j.phrs.2016.01.001.
Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–83. https://doi.org/10.1038/onc.2011.384.
Kelland LR. Preclinical perspectives on platinum resistance. Drugs. 2000;59:1–8. https://doi.org/10.2165/00003495-200059004-00001.
More SS, Akil O, Ianculescu AG, Geier EG, Lustig LR, Giacomini KM. Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J Neurosci. 2010;30:9500–9. https://doi.org/10.1523/JNEUROSCI.1544-10.2010.
Yamasaki M, Makino T, Masuzawa T, Kurokawa Y, Miyata H, Takiguchi S, et al. Role of multidrug resistance protein 2 (MRP2) in chemoresistance and clinical outcome in oesophageal squamous cell carcinoma. Br J Cancer. 2011;104:707–13. https://doi.org/10.1038/sj.bjc.6606071.
Gavande NS, VanderVere-Carozza PS, Hinshaw HD, Jalal SI, Sears CR, Pawelczak KS, et al. DNA repair targeted therapy: the past or future of cancer treatment?. Pharmacol Ther. 2016;160:65–83. https://doi.org/10.1016/j.pharmthera.2016.02.003.
Saldivar JS, Wu X, Follen M, Gershenson D. Nucleotide excision repair pathway review I: implications in ovarian cancer and platinum sensitivity. Gynecol Oncol. 2007;107:S56–71. https://doi.org/10.1016/j.ygyno.2007.07.043.
Baretti M, Le DT. DNA mismatch repair in cancer. Pharmacol Ther. 2018;189:45–62. https://doi.org/10.1016/j.pharmthera.2018.04.004.
Martinez-Rivera M, Siddik ZH. Resistance and gain-of-resistance phenotypes in cancers harboring wild-type p53. Biochem Pharmacol. 2012;83:1049–62. https://doi.org/10.1016/j.bcp.2011.12.026.
Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–16. https://doi.org/10.1038/nrm1962.
Zhang Y, Shen X. Heat shock protein 27 protects L929 cells from cisplatin-induced apoptosis by enhancing Akt activation and abating suppression of thioredoxin reductase activity. Clin Cancer Res. 2007;13:2855–64. https://doi.org/10.1158/1078-0432.CCR-06-2090.
Li D, Zhang M, Liu J, Li Z, Ni B. Potential therapies for HCC involving targeting the ferroptosis pathway. Am J Cancer Res. 2024;14:1446–65.
Wu K, Ma S, Xu X, Liu Y, Tian C, Zhang C, et al. Celecoxib and cisplatin dual-loaded microspheres synergistically enhance transarterial chemoembolization effect of hepatocellular carcinoma. Mater Today Bio. 2024;24:100927. https://doi.org/10.1016/j.mtbio.2023.100927.
Li Y, Zhang Y, Zhang S, Huang D, Li B, Liang G, et al. circRNA circARNT2 suppressed the sensitivity of hepatocellular carcinoma cells to cisplatin by targeting the miR-155-5p/PDK1 axis. Mol Ther Nucleic Acids. 2021;23:244–54. https://doi.org/10.1016/j.omtn.2020.08.037.
Maurer M, Su T, Saal LH, Koujak S, Hopkins BD, Barkley CR, et al. 3-Phosphoinositide-dependent kinase 1 potentiates upstream lesions on the phosphatidylinositol 3-kinase pathway in breast carcinoma. Cancer Res. 2009;69:6299–306. https://doi.org/10.1158/0008-5472.CAN-09-0820.
Li P, Song R, Yin F, Liu M, Liu H, Ma S, et al. circMRPS35 promotes malignant progression and cisplatin resistance in hepatocellular carcinoma. Mol Ther. 2022;30:431–47. https://doi.org/10.1016/j.ymthe.2021.08.027.
Duan JL, Chen W, Xie JJ, Zhang ML, Nie RC, Liang H, et al. A novel peptide encoded by N6-methyladenosine modified circMAP3K4 prevents apoptosis in hepatocellular carcinoma. Mol Cancer. 2022;21:93. https://doi.org/10.1186/s12943-022-01537-5.
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–6. https://doi.org/10.1038/17135.
Wang H, Liu M, Zeng X, Zheng Y, Wang Y, Zhou Y. Cell death affecting the progression of gastric cancer. Cell Death Discov. 2022;8:377. https://doi.org/10.1038/s41420-022-01161-8.
Gupta J, Ahmed AT, Tayyib NA, Zabibah RS, Shomurodov Q, Kadheim MN, et al. A state-of-art of underlying molecular mechanisms and pharmacological interventions/nanotherapeutics for cisplatin resistance in gastric cancer. Biomed Pharmacother. 2023;166:115337. https://doi.org/10.1016/j.biopha.2023.115337.
Sun Y, Ma J, Lin J, Sun D, Song P, Shi L, et al. Circular RNA circ_ASAP2 regulates drug sensitivity and functional behaviors of cisplatin-resistant gastric cancer cells by the miR-330-3p/NT5E axis. Anticancer Drugs. 2021;32:950–61. https://doi.org/10.1097/CAD.0000000000001087.
Jiao Y, Fu Y, Gong Y, Wang G, Chen S, Cai G, et al. Circ_0067997 boosted the growth while repressed the apoptosis of SGC-7901/DDP cells via repressing miR-615-5p/AKT1 pathway. Cancer Biomark. 2023;37:27–38. https://doi.org/10.3233/CBM-220145.
Shang Z, Luo Z, Wang Y, Liu Q, Xin Y, Zhang M, et al. CircHIPK3 contributes to cisplatin resistance in gastric cancer by blocking autophagy-dependent ferroptosis. J Cell Physiol. 2023;238:2407–24. https://doi.org/10.1002/jcp.31093.
Chen XY, Yang YL, Yu Y, Chen ZY, Fan HN, Zhang J, et al. CircUGGT2 downregulation by METTL14-dependent m(6)A modification suppresses gastric cancer progression and cisplatin resistance through interaction with miR-186-3p/MAP3K9 axis. Pharmacol Res. 2024;204:107206. https://doi.org/10.1016/j.phrs.2024.107206.
Mao C, Zeng X, Zhang C, Yang Y, Xiao X, Luan S, et al. Mechanisms of pharmaceutical therapy and drug resistance in esophageal cancer. Front Cell Dev Biol. 2021;9:612451. https://doi.org/10.3389/fcell.2021.612451.
Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, et al. Oesophageal cancer. Nat Rev Dis Prim. 2017;3:17048. https://doi.org/10.1038/nrdp.2017.48.
Yamada M, Tanaka K, Yamamoto K, Matsumoto H, Yamasaki M, Yamashita K, et al. Association between circ_0004365 and cisplatin resistance in esophageal squamous cell carcinoma. Oncol Lett. 2023;26:467. https://doi.org/10.3892/ol.2023.14054.
Liu Z, Gu S, Wu K, Li L, Dong C, Wang W, et al. CircRNA-DOPEY2 enhances the chemosensitivity of esophageal cancer cells by inhibiting CPEB4-mediated Mcl-1 translation. J Exp Clin Cancer Res. 2021;40:361. https://doi.org/10.1186/s13046-021-02149-5.
Zhang Z, Zhou Q, Luo F, Zhou R, Xu J, Xiao J, et al. Circular RNA circ-CHI3L1.2 modulates cisplatin resistance of osteosarcoma cells via the miR-340-5p/LPAATbeta axis. Hum Cell. 2021;34:1558–68. https://doi.org/10.1007/s13577-021-00564-6.
Amawi H, Sim HM, Tiwari AK, Ambudkar SV, Shukla S. ABC transporter-mediated multidrug-resistant cancer. Adv Exp Med Biol. 2019;1141:549–80. https://doi.org/10.1007/978-981-13-7647-4_12.
Si X, Su X, Lin W, Xu J, Huang W, Chen F, et al. Circ_ZNF778_006 promoted ESCC progression by upregulating HIF-1alpha expression via sponging miR-18b-5p. Sci Rep. 2023;13:19363. https://doi.org/10.1038/s41598-023-46832-3.
Shi Y, Gilkes DM. HIF-1 and HIF-2 in cancer: structure, regulation, and therapeutic prospects. Cell Mol Life Sci. 2025;82:44. https://doi.org/10.1007/s00018-024-05537-0.
Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmstrom PU, Choi W, et al. Bladder cancer. Lancet. 2016;388:2796–810. https://doi.org/10.1016/S0140-6736(16)30512-8.
Yang X, Ye T, Liu H, Lv P, Duan C, Wu X, et al. Expression profiles, biological functions and clinical significance of circRNAs in bladder cancer. Mol Cancer. 2021;20:4. https://doi.org/10.1186/s12943-020-01300-8.
Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin. 2020;70:404–23. https://doi.org/10.3322/caac.21631.
Wei W, Liu K, Huang X, Tian S, Wang H, Zhang C, et al. EIF4A3-mediated biogenesis of circSTX6 promotes bladder cancer metastasis and cisplatin resistance. J Exp Clin Cancer Res. 2024;43:2. https://doi.org/10.1186/s13046-023-02932-6.
Verstrepen L, Verhelst K, van Loo G, Carpentier I, Ley SC, Beyaert R. Expression, biological activities and mechanisms of action of A20 (TNFAIP3). Biochem Pharmacol. 2010;80:2009–20. https://doi.org/10.1016/j.bcp.2010.06.044.
Wei W, Sun J, Zhang H, Xiao X, Huang C, Wang L, et al. Circ0008399 interaction with WTAP promotes assembly and activity of the m(6)A methyltransferase complex and promotes cisplatin resistance in bladder cancer. Cancer Res. 2021;81:6142–56. https://doi.org/10.1158/0008-5472.CAN-21-1518.
Xu C, Zhou J, Zhang X, Kang X, Liu S, Song M, et al. N(6)-methyladenosine-modified circ_104797 sustains cisplatin resistance in bladder cancer through acting as RNA sponges. Cell Mol Biol Lett. 2024;29:28. https://doi.org/10.1186/s11658-024-00543-3.
Ju M, Wu W, Qu J, Sun Y, Li J. Downregulation of circular RNA hsa_circ_0087856 sensitizes bladder cancer cells to cisplatin through targeting miR-1184/CITED2 signaling. Environ Mol Mutagen. 2023;64:342–53. https://doi.org/10.1002/em.22561.
Zhang H, Xiao X, Wei W, Huang C, Wang M, Wang L, et al. CircLIFR synergizes with MSH2 to attenuate chemoresistance via MutSalpha/ATM-p73 axis in bladder cancer. Mol Cancer. 2021;20:70. https://doi.org/10.1186/s12943-021-01360-4.
Yoshida K, Ozaki T, Furuya K, Nakanishi M, Kikuchi H, Yamamoto H, et al. ATM-dependent nuclear accumulation of IKK-alpha plays an important role in the regulation of p73-mediated apoptosis in response to cisplatin. Oncogene. 2008;27:1183–8. https://doi.org/10.1038/sj.onc.1210722.
Lyu F, Huang S, Yan Z, He Q, Liu C, Cheng L, et al. CircUGGT2 facilitates progression and cisplatin resistance of bladder cancer through nonhomologous end-joining pathway. Cell Signal. 2024;119:111164. https://doi.org/10.1016/j.cellsig.2024.111164.
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. https://doi.org/10.3322/caac.21763.
Marchetti C, De Felice F, Romito A, Iacobelli V, Sassu CM, Corrado G, et al. Chemotherapy resistance in epithelial ovarian cancer: mechanisms and emerging treatments. Semin Cancer Biol. 2021;77:144–66. https://doi.org/10.1016/j.semcancer.2021.08.011.
Zhu Y, Liang L, Zhao Y, Li J, Zeng J, Yuan Y, et al. CircNUP50 is a novel therapeutic target that promotes cisplatin resistance in ovarian cancer by modulating p53 ubiquitination. J Nanobiotechnol. 2024;22:35.https://doi.org/10.1186/s12951-024-02295-w.
Li H, Lin R, Zhang Y, Zhu Y, Huang S, Lan J, et al. N6-methyladenosine-modified circPLPP4 sustains cisplatin resistance in ovarian cancer cells via PIK3R1 upregulation. Mol Cancer. 2024;23:5. https://doi.org/10.1186/s12943-023-01917-5.
Fu L, Zhang D, Yi N, Cao Y, Wei Y, Wang W, et al. Circular RNA circPBX3 promotes cisplatin resistance of ovarian cancer cells via interacting with IGF2BP2 to stabilize ATP7A mRNA expression. Hum Cell. 2022;35:1560–76. https://doi.org/10.1007/s13577-022-00748-8.
Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8:761–73. https://doi.org/10.7150/jca.17648.
Li H, Luo F, Jiang X, Zhang W, Xiang T, Pan Q, et al. CircITGB6 promotes ovarian cancer cisplatin resistance by resetting tumor-associated macrophage polarization toward the M2 phenotype. J Immunother Cancer. 2022;10. https://doi.org/10.1136/jitc-2021-004029.
Arbyn M, Weiderpass E, Bruni L, de Sanjose S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8:e191–203. https://doi.org/10.1016/S2214-109X(19)30482-6.
Zhao N, Li Y, Chen X, Ma J, Luo W, Li Y. Evaluating the clinical efficacy and safety of concurrent chemoradiotherapy with cisplatin and nab-paclitaxel in postoperative early-stage cervical cancer. J Cancer Res Clin Oncol. 2024;150:233. https://doi.org/10.1007/s00432-024-05764-9.
Wu P, Qin J, Liu L, Tan W, Lei L, Zhu J. circEPSTI1 promotes tumor progression and cisplatin resistance via upregulating MSH2 in cervical cancer. Aging. 2022;14:5406–16. https://doi.org/10.18632/aging.204152.
Chen Z, Xu Z, Wang Q, Wang L, Zhang H, Wang W, et al. Exosome-delivered circRNA circSYT15 contributes to cisplatin resistance in cervical cancer cells through the miR-503-5p/RSF1 axis. Cell Cycle. 2023;22:2211–28. https://doi.org/10.1080/15384101.2023.2281768.
Ma T, Guo J, Han J, Li L, Ren Y, Huang J, et al. Circ_0001589/miR-1248/HMGB1 axis enhances EMT-mediated metastasis and cisplatin resistance in cervical cancer. Mol Carcinog. 2023;62:1645–58. https://doi.org/10.1002/mc.23605.
Lv G, Yang M, Gai K, Jia Q, Wang Z, Wang B, et al. Multiple functions of HMGB1 in cancer. Front Oncol. 2024;14:1384109. https://doi.org/10.3389/fonc.2024.1384109.
Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization Classification of Lung Tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–60. https://doi.org/10.1097/JTO.0000000000000630.
Lim W, Ridge CA, Nicholson AG, Mirsadraee S. The 8(th) lung cancer TNM classification and clinical staging system: review of the changes and clinical implications. Quant Imaging Med Surg. 2018;8:709–18. https://doi.org/10.21037/qims.2018.08.02.
Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52:103–9. https://doi.org/10.1016/j.semcancer.2017.11.019.
Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5:288–300. https://doi.org/10.21037/tlcr.2016.06.07.
Greenhalgh J, Dwan K, Boland A, Bates V, Vecchio F, Dundar Y, et al. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst Rev. 2016;CD010383. https://doi.org/10.1002/14651858.CD010383.pub2.
Kenmotsu H, Ohde Y, Wakuda K, Nakashima K, Omori S, Ono A, et al. Survival data for postoperative adjuvant chemotherapy comprising cisplatin plus vinorelbine after complete resection of non-small cell lung cancer. Cancer Chemother Pharmacol. 2017;80:609–14. https://doi.org/10.1007/s00280-017-3400-z.
Xie H, Yao J, Wang Y, Ni B. Exosome-transmitted circVMP1 facilitates the progression and cisplatin resistance of non-small cell lung cancer by targeting miR-524-5p-METTL3/SOX2 axis. Drug Deliv. 2022;29:1257–71. https://doi.org/10.1080/10717544.2022.2057617.
Zhang S, Xiong X, Sun Y. Functional characterization of SOX2 as an anticancer target. Signal Transduct Target Ther. 2020;5:135. https://doi.org/10.1038/s41392-020-00242-3.
He H, Li T. Hsa_circ_0000190 Promotes NSCLC Cell Resistance to Cisplatin via the Modulation of the miR-1253/IL-6 Axis. Anal Cell Pathol. 2024;2024:6647810. https://doi.org/10.1155/2024/6647810.
Lu H, Kong J, Cai S, Huang H, Luo J, Liu L. Hsa_circ_0096157 silencing suppresses autophagy and reduces cisplatin resistance in non-small cell lung cancer by weakening the Nrf2/ARE signaling pathway. Mol Biol Rep. 2024;51:703. https://doi.org/10.1007/s11033-024-09552-z.
Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, et al. The 2021 WHO Classification of Lung Tumors: impact of advances since 2015. J Thorac Oncol. 2022;17:362–87. https://doi.org/10.1016/j.jtho.2021.11.003.
Wei X, Li X, Hu S, Cheng J, Cai R. Regulation of ferroptosis in lung adenocarcinoma. Int J Mol Sci. 2023;24. https://doi.org/10.3390/ijms241914614.
Yu N, Gong H, Chen W, Peng W. CircRNA ZKSCAN1 promotes lung adenocarcinoma progression by miR-185-5p/TAGLN2 axis. Thorac Cancer. 2023;14:1467–76. https://doi.org/10.1111/1759-7714.14889.
Cao L, Zhou X, Ding X, Gao D. Knockdown of circ‑PVT1 inhibits the progression of lung adenocarcinoma and enhances the sensitivity to cisplatin via the miR‑429/FOXK1 signaling axis. Mol Med Rep. 2021;24. https://doi.org/10.3892/mmr.2021.12323.
Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80. https://doi.org/10.1016/S0140-6736(19)30956-0.
Tian X, Zhu Q, Zhang Z. Efficacy and safety of weekly versus triweekly cisplatin treatment concomitant with radiotherapy for locally advanced nasopharyngeal carcinoma: a systematic review and pooled analysis. Front Pharmacol. 2022;13:999027. https://doi.org/10.3389/fphar.2022.999027.
Deng G, Wang F, Song Y. Circular RNA SET domain protein 3 promotes nasopharyngeal carcinoma proliferation, cisplatin resistance, and protein kinase B/mammalian target of rapamycin pathway activation by modulating microRNA-147a expression. Bioengineered. 2022;13:5843–54. https://doi.org/10.1080/21655979.2022.2036907.
Hong X, Li Q, Li J, Chen K, He Q, Zhao Y, et al. CircIPO7 promotes nasopharyngeal carcinoma metastasis and cisplatin chemoresistance by facilitating YBX1 nuclear localization. Clin Cancer Res. 2022;28:4521–35. https://doi.org/10.1158/1078-0432.CCR-22-0991.
Guccini I, Revandkar A, D’Ambrosio M, Colucci M, Pasquini E, Mosole S, et al. Senescence reprogramming by TIMP1 deficiency promotes prostate cancer metastasis. Cancer Cell. 2021;39:68–82.e9. https://doi.org/10.1016/j.ccell.2020.10.012.
Li Q, Zhao YH, Xu C, Liang YL, Zhao Y, He QM, et al. Chemotherapy-induced senescence reprogramming promotes nasopharyngeal carcinoma metastasis by circRNA-mediated PKR activation. Adv Sci. 2023;10:e2205668. https://doi.org/10.1002/advs.202205668.
Hattinger CM, Casotti C, Patrizio MP, Luppi S, Fantoni L, Scotlandi K, et al. Pharmacogenomic profiling of cisplatin-resistant and -sensitive human osteosarcoma cell lines by multimodal targeted next generation sequencing. Int J Mol Sci. 2022;23. https://doi.org/10.3390/ijms231911787.
Lilienthal I, Herold N. Targeting molecular mechanisms underlying treatment efficacy and resistance in osteosarcoma: a review of current and future strategies. Int J Mol Sci. 2020;21. https://doi.org/10.3390/ijms21186885.
Pan Y, Lin Y, Mi C. Cisplatin-resistant osteosarcoma cell-derived exosomes confer cisplatin resistance to recipient cells in an exosomal circ_103801-dependent manner. Cell Biol Int. 2021;45:858–68. https://doi.org/10.1002/cbin.11532.
Zhang J, Ma X, Zhou R, Zhou Y. TRPS1 and YAP1 regulate cell proliferation and drug resistance of osteosarcoma via competitively binding to the target of circTADA2A–miR-129-5p. Onco Targets Ther. 2020;13:12397–407. https://doi.org/10.2147/OTT.S276953.
Ieiri I, Takane H, Otsubo K. The MDR1 (ABCB1) gene polymorphism and its clinical implications. Clin Pharmacokinet. 2004;43:553–76. https://doi.org/10.2165/00003088-200443090-00001.
Li S, Liu F, Zheng K, Wang W, Qiu E, Pei Y, et al. CircDOCK1 promotes the tumorigenesis and cisplatin resistance of osteogenic sarcoma via the miR-339-3p/IGF1R axis. Mol Cancer. 2021;20:161. https://doi.org/10.1186/s12943-021-01453-0.
Wang B, Yan L, Shi W, Xie H, Chen R, Shao Y, et al. CircRNA PVT1 promotes proliferation and chemoresistance of osteosarcoma cells via the miR-24-3p/KLF8 axis. Int J Clin Oncol. 2022;27:811–22. https://doi.org/10.1007/s10147-022-02122-y.
Kumar S, Behera A, Saha P, Kumar Srivastava A. The role of Kruppel-like factor 8 in cancer biology: current research and its clinical relevance. Biochem Pharmacol. 2021;183:114351. https://doi.org/10.1016/j.bcp.2020.114351.
Ma Y, Gao J, Guo H. Circ_0000140 alters miR-527/SLC7A11-mediated ferroptosis to influence oral squamous cell carcinoma cell resistance to DDP. Pharmgenom Pers Med. 2023;16:1079–89. https://doi.org/10.2147/PGPM.S426205.
Sun G, Tian J, Xiao Y, Zeng Y. Circular RNA circ_0005667 promotes cisplatin resistance of endometrial carcinoma cells by regulating IGF2BP1 through miR-145-5p. Anticancer Drugs. 2023;34:816–26. https://doi.org/10.1097/CAD.0000000000001479.
Gola AM, Bucci-Munoz M, Rigalli JP, Ceballos MP, Ruiz ML. Role of the RNA binding protein IGF2BP1 in cancer multidrug resistance. Biochem Pharmacol. 2024;230:116555. https://doi.org/10.1016/j.bcp.2024.116555.
Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Prim. 2020;6:92. https://doi.org/10.1038/s41572-020-00224-3.
Wang T, Xin C, Zhang S, Tian X, Hu Y, Wang Y, et al. Circular RNA from tyrosylprotein sulfotransferase 2 gene inhibits cisplatin sensitivity in head and neck squamous cell carcinoma by sponging miR-770-5p and interacting with nucleolin. Cancers. 2023;15. https://doi.org/10.3390/cancers15225351.
Cao L, Zhu Y, Wang W, Wang G, Zhang S, Cheng H. Emerging nano-based strategies against drug resistance in tumor chemotherapy. Front Bioeng Biotechnol. 2021;9:798882. https://doi.org/10.3389/fbioe.2021.798882.
Dasari S, Njiki S, Mbemi A, Yedjou CG, Tchounwou PB. Pharmacological effects of cisplatin combination with natural products in cancer chemotherapy. Int J Mol Sci. 2022;23. https://doi.org/10.3390/ijms23031532.
Li J, Ye Z, Hu X, Hou S, Hang Q. Prognostic, diagnostic, and clinicopathological significance of circular RNAs in pancreatic cancer: a systematic review and meta-analysis. Cancers. 2022;14. https://doi.org/10.3390/cancers14246187.
Xu L, Chen Y, Ye J, Fan M, Weng G, Shen Y, et al. Optical nanobiosensor based on surface-enhanced raman spectroscopy and catalytic hairpin assembly for early-stage lung cancer detection via blood circular RNA. ACS Sens. 2024;9:2020–30. https://doi.org/10.1021/acssensors.3c02810.
Bu T, Yang Z, Zhao J, Gao Y, Li F, Yang R. Expanding the potential of circular RNA (circRNA) vaccines: a promising therapeutic approach. Int J Mol Sci. 2025;26. https://doi.org/10.3390/ijms26010379.
Wang F, Cai G, Wang Y, Zhuang Q, Cai Z, Li Y, et al. Circular RNA-based neoantigen vaccine for hepatocellular carcinoma immunotherapy. MedComm. 2024;5:e667. https://doi.org/10.1002/mco2.667.
Author information
Authors and Affiliations
Contributions
Jiawen Zhang: proposed and designed the article, writing—original draft preparation. Qiwen Yu: prepared the tables and figures, writing—initial revision. Weijin Zhu: contributed to the critical revision of the manuscript. Xiaochun Wu: edited, supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Unethical issues (including plagiarism, informed consent, misconduct, data fabrication, forgery, duplication and/or submission, and redundancy) have been investigated by the authors.
Consent for publication
All authors consent for this submission.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Yu, Q., Zhu, W. et al. Recent advances in the role of circRNA in cisplatin resistance in tumors. Cancer Gene Ther 32, 497–506 (2025). https://doi.org/10.1038/s41417-025-00899-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41417-025-00899-4