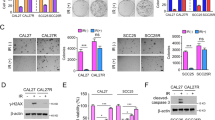
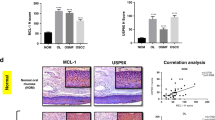
Abstract
Oral squamous cell carcinoma (OSCC) represents the most common type of malignant oral tumor, with a high prevalence globally. Despite continual advancements in OSCC treatment, the 5-year survival rate remains around 50%, highlighting an urgent need for the development of new and effective therapeutic strategies. Here, we focused on myeloid leukemia 1 (Mcl-1), a well-known oncogenic driver in various human cancers, and systematically explored the therapeutic potential of oleuropein (Ole) through in vitro and in vivo analyses. Our findings demonstrated that Ole suppressed OSCC cell viability dose-dependently. Mechanistically, Ole facilitated β-TRCP-mediated ubiquitination of Mcl-1 by inhibiting the Akt-GSK3β-Mcl-1 pathway and enhancing the collaboration between β-TRCP and Mcl-1, ultimately leading to Mcl-1 degradation. Furthermore, the knockdown of β-TRCP mitigated the inhibitory effects of Ole on OSCC cells. In agreement with our cell-based experiments, animal studies showed that Ole treatment significantly delayed tumor growth without causing toxicity to vital organs. Additionally, whether used alone or combined with radiation, Ole effectively overcame radioresistance in OSCC cells. Our results suggest that Ole is a promising anti-tumor agent capable of treating OSCC by targeting Mcl-1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Data availability
The datasets used and analyzed in this study are available from the corresponding author (WL) upon request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Peng QS, Cheng YN, Zhang WB, Fan H, Mao QH, Xu P. circRNA_0000140 suppresses oral squamous cell carcinoma growth and metastasis by targeting miR-31 to inhibit Hippo signaling pathway. Cell Death Dis. 2020;11:112.
Liu S, Gao W, Lu Y, Zhou Q, Su R, Hasegawa T, et al. As a novel tumor suppressor, LHPP promotes apoptosis by inhibiting the PI3K/AKT signaling pathway in oral squamous cell carcinoma. Int J Biol Sci. 2022;18:491–506.
Goyal N, Hennessy M, Lehman E, Lin W, Agudo A, Ahrens W, et al. Risk factors for head and neck cancer in more and less developed countries: analysis from the INHANCE consortium. Oral Dis. 2023;29:1565–78.
Zhou Z, Han S, Liao J, Wang R, Yu X, Li M. Isoliquiritigenin inhibits oral squamous cell carcinoma and overcomes chemoresistance by destruction of survivin. Am J Chin Med. 2023;51:2221–41.
Fan T, Wang X, Zhang S, Deng P, Jiang Y, Liang Y, et al. NUPR1 promotes the proliferation and metastasis of oral squamous cell carcinoma cells by activating TFE3-dependent autophagy. Signal Transduct Target Ther. 2022;7:130.
Wang D, Qi H, Zhang H, Zhou W, Li Y, Li A, et al. TAF1L promotes development of oral squamous cell carcinoma via decreasing autophagy-dependent apoptosis. Int J Biol Sci. 2020;16:1180–93.
Chen YT, Hsieh MJ, Chen PN, Weng CJ, Yang SF, Lin CW. Erianin induces apoptosis and autophagy in oral squamous cell carcinoma cells. Am J Chin Med. 2020;48:183–200.
Zhang J, Xu HX, Zhu JQ, Dou YX, Xian YF, Lin ZX. Natural Nrf2 inhibitors: a review of their potential for cancer treatment. Int J Biol Sci. 2023;19:3029–41.
Hong Z, Tang P, Liu B, Ran C, Yuan C, Zhang Y, et al. Ferroptosis-related genes for overall survival prediction in patients with colorectal cancer can be inhibited by gallic acid. Int J Biol Sci. 2021;17:942–56.
Wei W, Rasul A, Sadiqa A, Sarfraz I, Hussain G, Nageen B, et al. Curcumol: from plant roots to cancer roots. Int J Biol Sci. 2019;15:1600–9.
Liu Y, Shi C, He Z, Zhu F, Wang M, He R, et al. Inhibition of PI3K/AKT signaling via ROS regulation is involved in Rhein-induced apoptosis and enhancement of oxaliplatin sensitivity in pancreatic cancer cells. Int J Biol Sci. 2021;17:589–602.
Liu W, Li W, Liu H, Yu X. Xanthohumol inhibits colorectal cancer cells via downregulation of Hexokinases II-mediated glycolysis. Int J Biol Sci. 2019;15:2497–508.
Zhou L, Li M, Yu X, Gao F, Li W. Repression of hexokinases II-mediated glycolysis contributes to piperlongumine-induced tumor suppression in non-small cell lung cancer cells. Int J Biol Sci. 2019;15:826–37.
Marrone G, Urciuoli S, Candi E, Bernini R, Vanni G, Masci C, et al. Biological activities of molecules derived from Olea europaea L. tested in vitro. Life. 2023;14:49.
Cerri L, Parri S, Dias MC, Fabiano A, Romi M, Cai G, et al. Olive leaf extracts from three italian olive cultivars exposed to drought stress differentially protect cells against oxidative stress. Antioxidants. 2024;13:77.
Infante R, Infante M, Pastore D, Pacifici F, Chiereghin F, Malatesta G, et al. An appraisal of the oleocanthal-rich extra virgin olive oil (EVOO) and its potential anticancer and neuroprotective properties. Int J Mol Sci. 2023;24:17323.
Park SM, Kim DY, Lee KH, Shin YI, Han SC, Kwon SM. Anti-tumor efficacy of oleuropein-loaded ZnO/Au mesoporous silica nanoparticle in 5-FU-resistant colorectal cancer cells. Int J Nanomedicine. 2024;19:2675–90.
Hong Z, Lu Y, Liu B, Ran C, Lei X, Wang M, et al. Glycolysis, a new mechanism of oleuropein against liver tumor. Phytomedicine. 2023;114:154770.
Filardo S, Roberto M, Di Risola D, Mosca L, Di Pietro M, Sessa R. Olea europaea L-derived secoiridoids: beneficial health effects and potential therapeutic approaches. Pharmacol Ther. 2024;254:108595.
Shamshoum H, Vlavcheski F, Tsiani E. Anticancer effects of oleuropein. Biofactors. 2017;43:517–28.
Ercelik M, Tekin C, Parin FN, Mutlu B, Dogan HY, Tezcan G, et al. Co-loading of Temozolomide with Oleuropein or rutin into polylactic acid core-shell nanofiber webs inhibit glioblastoma cell by controlled release. Int J Biol Macromol. 2023;253:126722.
Kapoor I, Bodo J, Hill BT, Hsi ED, Almasan A. Targeting BCL-2 in B-cell malignancies and overcoming therapeutic resistance. Cell Death Dis. 2020;11:941.
Ren H, Koo J, Guan B, Yue P, Deng X, Chen M, et al. The E3 ubiquitin ligases β-TrCP and FBXW7 cooperatively mediates GSK3-dependent Mcl-1 degradation induced by the Akt inhibitor API-1, resulting in apoptosis. Mol Cancer. 2013;12:146.
Wang H, Guo M, Wei H, Chen Y. Targeting MCL-1 in cancer: current status and perspectives. J Hematol Oncol. 2021;14:67.
Luo F, Lu FT, Qiu MZ, Zhou T, Ma WJ, Luo M, et al. Gemcitabine and APG-1252, a novel small molecule inhibitor of BCL-2/BCL-XL, display a synergistic antitumor effect in nasopharyngeal carcinoma through the JAK-2/STAT3/MCL-1 signaling pathway. Cell Death Dis. 2021;12:772.
Floros KV, Jacob S, Kurupi R, Fairchild CK, Hu B, Puchalapalli M, et al. Targeting transcription of MCL-1 sensitizes HER2-amplified breast cancers to HER2 inhibitors. Cell Death Dis. 2021;12:179.
Li M, Gao F, Li X, Gan Y, Han S, Yu X, et al. Stabilization of MCL-1 by E3 ligase TRAF4 confers radioresistance. Cell Death Dis. 2022;13:1053.
Li Y, Lee HH, Jiang VC, Che Y, McIntosh J, Jordan A, et al. Potentiation of apoptosis in drug-resistant mantle cell lymphoma cells by MCL-1 inhibitor involves downregulation of inhibitor of apoptosis proteins. Cell Death Dis. 2023;14:714.
Fitzgerald MC, O’Halloran PJ, Kerrane SA, Connolly TNC, Murphy NMC. BM. The identification of BCL-XL and MCL-1 as key anti-apoptotic proteins in medulloblastoma that mediate distinct roles in chemotherapy resistance. Cell Death Dis. 2023;14:705.
Valentini E, Di Martile M, Brignone M, Di Caprio M, Manni I, Chiappa M, et al. Bcl-2 family inhibitors sensitize human cancer models to therapy. Cell Death Dis. 2023;14:441.
Feng Q, Yang P, Wang H, Li C, Hasegawa T, Liu Z, et al. ID09, a newly-designed tubulin inhibitor, regulating the proliferation, migration, EMT process and apoptosis of oral squamous cell carcinoma. Int J Biol Sci. 2022;18:473–90.
Kim YI, Park SW, Choi IH, Lee JH, Woo HJ, Kim Y. Effect of Orostachys japonicus on cell growth and apoptosis in human hepatic stellate cell line LX2. Am J Chin Med. 2011;39:601–13.
Takagi S, Nakajima M, Koike S, Takami M, Sugiura Y, Sakata S, et al. Frequent copy number gain of MCL1 is a therapeutic target for osteosarcoma. Oncogene. 2025;44:794–804.
Koleci N, Wu Y, Wehner NA, Rajak J, Mittapalli VR, Mergner J, et al. Oncogenic and microenvironmental signals drive cell type specific apoptosis resistance in juvenile myelomonocytic leukemia. Cell Death Dis. 2025;16:165.
Yu X, Zhou L, Liu W, Liu L, Gao F, Li W, et al. Skp2 stabilizes Mcl-1 and confers radioresistance in colorectal cancer. Cell Death Dis. 2022;13:249.
Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–60.
Li XY, Gao F, Wang XC, Liu LL, Gan Y, Han SZ, et al. Curcumol exerts antitumor effect via inhibiting EGFR-Akt-Mcl-1 signaling. Am J Chin Med. 2023;51:741–60.
Park Y, Cho YJ, Sung N, Park MJ, Guan X, Gibbons WE, et al. Oleuropein suppresses endometriosis progression and improves the fertility of mice with endometriosis. J Biomed Sci. 2022;29:100.
Zhang Z, Zhao H, Wang A. Oleuropein alleviates gestational diabetes mellitus by activating AMPK signaling. Endocr Connect. 2021;10:45–53.
Choupani J, Alivand MR, M Derakhshan S, Zaeifizadeh M, S Khaniani M. Oleuropein inhibits migration ability through suppression of epithelial-mesenchymal transition and synergistically enhances doxorubicin-mediated apoptosis in MCF-7 cells. J Cell Physiol. 2019;234:9093–104.
Xing Y, Cui D, Wang S, Wang P, Xing X, Li H. Oleuropein represses the radiation resistance of ovarian cancer by inhibiting hypoxia and microRNA-299-targetted heparanase expression. Food Funct. 2017;8:2857–64.
Papakonstantinou A, Koumarianou P, Diamantakos P, Melliou E, Magiatis P, Boleti H. A systematic ex-vivo study of the anti-proliferative/cytotoxic bioactivity of major olive secoiridoids’ double combinations and of total olive oil phenolic extracts on multiple cell-culture based cancer models highlights synergistic effects. Nutrients. 2023;15:2538.
Blanco E, Silva-Pilipich N, Bocanegra A, Chocarro L, Procopio A, Ausín K, et al. Oleuropein-driven reprogramming of the myeloid cell compartment to sensitise tumours to PD-1/PD-L1 blockade strategies. Br J Cancer. 2024;130:869–79.
Gioti K, Papachristodoulou A, Benaki D, Aligiannis N, Skaltsounis AL, Mikros E, et al. Assessment of the nutraceutical effects of oleuropein and the cytotoxic effects of adriamycin, when administered alone and in combination, in MG-63 human osteosarcoma cells. Nutrients. 2021;13:354.
Xu T, Xiao D. Oleuropein enhances radiation sensitivity of nasopharyngeal carcinoma by downregulating PDRG1 through HIF1α-repressed microRNA-519d. J Exp Clin Cancer Res. 2017;36:3.
Grawish ME, Zyada MM, Zaher AR. Inhibition of 4-NQO-induced F433 rat tongue carcinogenesis by oleuropein-rich extract. Med Oncol. 2011;28:1163–8.
Xu T, Liu X. Oleuropein inhibits invasion of squamous cell carcinoma of the head and neck through TGF-beta1 signaling pathway. BMC Cancer. 2022;22:942.
Karpel-Massler G, Horst BA, Shu C, Chau L, Tsujiuchi T, Bruce JN, et al. A synthetic cell-penetrating dominant-negative ATF5 peptide exerts anticancer activity against a broad spectrum of treatment-resistant cancers. Clin Cancer Res. 2016;22:4698–711.
Tian H, Qiang T, Wang J, Ji L, Li B. Simvastatin regulates the proliferation, apoptosis, migration and invasion of human acute myeloid leukemia cells via miR-19a-3p/HIF-1α axis. Bioengineered. 2021;12:11898–908.
Lin W, Sun J, Sadahira T, Xu N, Wada K, Liu C, et al. Discovery and validation of nitroxoline as a novel STAT3 inhibitor in drug-resistant urothelial bladder cancer. Int J Biol Sci. 2021;17:3255–67.
Wu T, Yu B, Xu Y, Du Z, Zhang Z, Wang Y, et al. Discovery of selective and potent macrocyclic CDK9 inhibitors for the treatment of osimertinib-resistant non-small-cell lung cancer. J Med Chem. 2023;66:15340–61.
Tantawy SI, Timofeeva N, Sarkar A, Gandhi V. Targeting MCL-1 protein to treat cancer: opportunities and challenges. Front Oncol. 2023;13:1226289.
Xie L, Zeng Y, Dai Z, He W, Ke H, Lin Q, et al. Chemical and genetic inhibition of STAT3 sensitizes hepatocellular carcinoma cells to sorafenib induced cell death. Int J Biol Sci. 2018;14:577–85.
Sneyers F, Kerkhofs M, Speelman-Rooms F, Welkenhuyzen K, La Rovere R, Shemy A, et al. Intracellular BAPTA directly inhibits PFKFB3, thereby impeding mTORC1-driven Mcl-1 translation and killing MCL-1-addicted cancer cells. Cell Death Dis. 2023;14:600.
Liu Y, Lei P, Qiao H, Sun K, Lu X, Bao F, et al. miR-9 enhances the chemosensitivity of AML cells to daunorubicin by targeting the EIF5A2/MCL-1 axis. Int J Biol Sci. 2019;15:579–86.
Naghdi S, Mishra P, Roy SS, Weaver D, Walter L, Davies E, et al. VDAC2 and Bak scarcity in liver mitochondria enables targeting hepatocarcinoma while sparing hepatocytes. Nat Commun. 2025;16:2416.
Liang YY, Niu FY, Xu AA, Jiang LL, Liu CS, Liang HP, et al. Increased MCL-1 synthesis promotes irradiation-induced nasopharyngeal carcinoma radioresistance via regulation of the ROS/AKT loop. Cell Death Dis. 2022;13:131.
Wu T, Huang J, Zhang X, Ma F, Yu S, Liu Y, et al. Rational design of a potent, selective, and metabolically stable CDK9 inhibitor to counteract osimertinib resistance through Mcl-1 suppression and enhanced BRD4 Co-targeting. J Med Chem. 2025;68:4929–50.
Tantawy SI, Sarkar A, Hubner S, Tan Z, Wierda WG, Eldeib A, et al. Mechanisms of MCL-1 protein stability induced by MCL-1 antagonists in B-cell malignancies. Clin Cancer Res. 2023;29:446–57.
Pan P, Ge W, Lei Z, Luo W, Liu Y, Guan Z, et al. SARS-CoV-2 N protein enhances the anti-apoptotic activity of MCL-1 to promote viral replication. Signal Transduct Target Ther. 2023;8:194.
Gores GJ, Kaufmann SH. Selectively targeting Mcl-1 for the treatment of acute myelogenous leukemia and solid tumors. Genes Dev. 2012;26:305–11.
Xu L, Liu Q, Liu H, Fan F, Li P, Yue S, et al. Disrupting CCDC137-mediated LZTS2 and beta-TrCP interaction in the nucleus inhibits hepatocellular carcinoma development via beta-catenin and AKT. Cell Death Differ. 2025;32:134–48.
Merino D, Whittle JR, Vaillant F, Serrano A, Gong JN, Giner G, et al. Synergistic action of the MCL-1 inhibitor S63845 with current therapies in preclinical models of triple-negative and HER2-amplified breast cancer. Sci Transl Med. 2017;9:eaam7049.
Bolomsky A, Vogler M, Köse MC, Heckman CA, Ehx G, Ludwig H, et al. MCL-1 inhibitors, fast-lane development of a new class of anti-cancer agents. J Hematol Oncol. 2020;13:173.
Williams MM, Elion DL, Rahman B, Hicks DJ, Sanchez V, Cook RS. Therapeutic inhibition of Mcl-1 blocks cell survival in estrogen receptor-positive breast cancers. Oncotarget. 2019;10:5389–402.
Wang Y, Han X, Wan X, Niu F, Zhou C. β-Escin: an updated review of its analysis, pharmacology, pharmacokinetics, and toxicity. Am J Chin Med. 2023;51:2095–120.
Gan Y, Li X, Han S, Zhou L, Li W. Targeting Mcl-1 degradation by bergenin inhibits tumorigenesis of colorectal cancer cells. Pharmaceuticals. 2023;16:241.
Chen CH, Hsu FT, Chen WL, Chen JH. Induction of apoptosis, inhibition of MCL-1, and VEGF-A expression are associated with the anti-cancer efficacy of magnolol combined with regorafenib in hepatocellular carcinoma. Cancers. 2021;13:2066.
Gao F, Yu X, Li M, Zhou L, Liu W, Li W, et al. Deguelin suppresses non-small cell lung cancer by inhibiting EGFR signaling and promoting GSK3β/FBW7-mediated Mcl-1 destabilization. Cell Death Dis. 2020;11:143.
Li M, Gao F, Yu X, Zhao Q, Zhou L, Liu W, et al. Promotion of ubiquitination-dependent survivin destruction contributes to xanthohumol-mediated tumor suppression and overcomes radioresistance in human oral squamous cell carcinoma. J Exp Clin Cancer Res. 2020;39:88.
Liao J, Qing X, Li X, Gan Y, Wang R, Han S, et al. TRAF4 regulates ubiquitination-modulated survivin turnover and confers radioresistance. Int J Biol Sci. 2024;20:182–99.
Dong X, Li X, Gan Y, Ding J, Wei B, Zhou L, et al. TRAF4-mediated ubiquitination-dependent activation of JNK/Bcl-xL drives radioresistance. Cell Death Dis. 2023;14:102.
Zhou Y, Li M, Yu X, Liu T, Li T, Zhou L, et al. Butein suppresses hepatocellular carcinoma growth via modulating Aurora B kinase activity. Int J Biol Sci. 2018;14:1521–34.
Funding
This work was supported by the Wisdom Accumulation and Talent Cultivation Project of the Third Xiangya Hospital of Central South University (BJ202203).
Author information
Authors and Affiliations
Contributions
Wen Liu, Song Peng, and Wei Li conceived and designed the project. Wen Liu, Song Peng, Jinzhuang Liao, Ruirui Wang, and Pengfei Guo administered the project and developed its methodology. Wen Liu, Song Peng, Jinzhuang Liao, Ruirui Wang, and Pengfei Guo analyzed and interpreted the data. Wen Liu and Song Peng wrote the original draft. Wei Li supervised the manuscript and acquired funding. All authors have read and agreed to the published version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal experiments were approved by the Institutional Animal Care and Use Committee, the Third Xiangya Hospital of Central South University (Changsha, China). All methods were performed in accordance with the relevant guidelines and regulations. Clinical trial number: not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, W., Peng, S., Liao, J. et al. Oleuropein regulates ubiquitination-mediated Mcl-1 turnover and exhibits antitumor activity. Cancer Gene Ther 32, 793–805 (2025). https://doi.org/10.1038/s41417-025-00921-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41417-025-00921-9