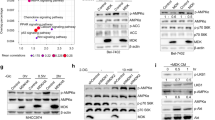
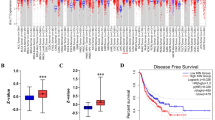
Abstract
Drug resistance exhibited by cancer cells remains one of the primary reasons for the failure of therapeutic approaches to increase the survival of cancer patients. Marginal improvement in therapeutic efficacy with current treatment approaches for non-small cell lung cancer (NSCLC) mandates new treatment strategies. Tax interacting Protein-1 (TIP1) is a radiation-inducible molecular target involved in various cancer pathways. TIP1 expression correlates with poor survival in NSCLC patients. Antibody blocking the functional domain of TIP1 reduced cell proliferation and sensitized cancer cells to radiation. A ten-fold increase in Midkine (MDK) was observed in the proteomic analysis of cells treated with anti-TIP1 antibody. Wnt signaling activation led to MDK upregulation at the mRNA and protein levels following TIP1 blockade. Genetic silencing of β-catenin abrogated the induction of MDK following anti-TIP1 antibody treatment. Inhibiting TIP1 along with MDK showed a reduction in the colony-forming capability of the cells, indicating that MDK upregulation might be a strategy employed by cancer cells to combat the anti-proliferative capabilities of the anti-TIP1 antibody. Co-targeting cell surface TIP1 and MDK may be an effective therapeutic strategy for NSCLC patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49.
Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res. 2015;4:36–54.
Rivera-Concepcion J, Uprety D, Adjei AA. Challenges in the use of targeted therapies in non-small cell lung cancer. Cancer Res Treat. 2022;54:315–29.
Vander Velde R, Yoon N, Marusyk V, Durmaz A, Dhawan A, Miroshnychenko D, et al. Resistance to targeted therapies as a multifactorial, gradual adaptation to inhibitor specific selective pressures. Nat Commun. 2020;11:2393.
Mohanty S, Ovee M, Banerjee M. PDZ domain recognition: Insight from Human Tax-Interacting Protein 1 (TIP-1) interaction with target proteins. Biology. 2015;4:88–103.
Yan H, Kapoor V, Nguyen K, Akers WJ, Li H, Scott J, et al. Anti-tax interacting protein-1 (TIP-1) monoclonal antibody targets human cancers. Oncotarget. 2016;7:43352–62.
Kapoor V, Singh AK, Rogers BE, Thotala D, Hallahan DE. PEGylated peptide to TIP1 is a novel targeting agent that binds specifically to various cancers in vivo. J Control Rel. 2019;298:194–201.
Lewis CD, Singh AK, Hsu FF, Thotala D, Hallahan DE, Kapoor V. Targeting a radiosensitizing antibody-drug conjugate to a radiation-inducible antigen. Clin Cancer Res. 2021;27:3224–33.
Johnson M, Sharma M, Jamieson C, Henderson JM, Mok MT, Bendall L, et al. Regulation of beta-catenin trafficking to the membrane in living cells. Cell Signal. 2009;21:339–48.
Kim WK, Kwon Y, Jang M, Park M, Kim J, Cho S, et al. beta-catenin activation down-regulates cell-cell junction-related genes and induces epithelial-to-mesenchymal transition in colorectal cancers. Sci Rep. 2019;9:18440.
Shah K, Kazi JU. Phosphorylation-dependent regulation of WNT/Beta-Catenin Signaling. Front Oncol. 2022;12:858782.
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, et al. Wnt/beta-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7:3.
Singh AK, Dadey DY, Rau MJ, Fitzpatrick J, Shah HK, Saikia M, et al. Blocking the functional domain of TIP1 by antibodies sensitizes cancer to radiation therapy. Biomed Pharmacother. 2023;166:115341.
Saikia M, Cheung N, Singh AK, Kapoor V. Role of Midkine in cancer drug resistance: regulators of its expression and its molecular targeting. Int J Mol Sci 2023;24:8739.
Cerezo-Wallis D, Contreras-Alcalde M, Troule K, Catena X, Mucientes C, Calvo TG, et al. Midkine rewires the melanoma microenvironment toward a tolerogenic and immune-resistant state. Nat Med. 2020;26:1865–77.
Ding L, Wang N, Wang Q, Fan X, Xin Y, Wang S. Midkine inhibition enhances anti-PD-1 immunotherapy in sorafenib-treated hepatocellular carcinoma via preventing immunosuppressive MDSCs infiltration. Cell Death Discov. 2023;9:92.
Huang WL, Hsu YC, Luo CW, Chang SJ, Hung YH, Lai CY, et al. Targeting the CDK7-MDK axis to suppresses irinotecan resistance in colorectal cancer. Life Sci 2024:122914.
Singh AK, Lewis CD, Boas C, Diebolder P, Jethva PN, Rhee A, et al. Development of a [89Zr]Zr-labeled human antibody using a novel phage-displayed human scFv Library. Clin Cancer Res. 2024;30:1293–306.
Nabbi A, Riabowol K. Rapid isolation of nuclei from cells in vitro. Cold Spring Harb Protoc. 2015;2015:769–72.
Stewart DJ. Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst. 2014;106:djt356.
Muto S, Enta A, Maruya Y, Inomata S, Yamaguchi H, Mine H, et al. Wnt/beta-Catenin signaling and resistance to immune checkpoint inhibitors: from non-small-cell lung cancer to other cancers. Biomedicines 2023;11:190.
Kanamori M, Sandy P, Marzinotto S, Benetti R, Kai C, Hayashizaki Y, et al. The PDZ protein tax-interacting protein-1 inhibits beta-catenin transcriptional activity and growth of colorectal cancer cells. J Biol Chem. 2003;278:38758–64.
Zhang J, Yan X, Shi C, Yang X, Guo Y, Tian C, et al. Structural basis of beta-catenin recognition by Tax-interacting protein-1. J Mol Biol. 2008;384:255–63.
Persad A, Venkateswaran G, Hao L, Garcia ME, Yoon J, Sidhu J, et al. Active beta-catenin is regulated by the PTEN/PI3 kinase pathway: a role for protein phosphatase PP2A. Genes Cancer. 2016;7:368–82.
Staal FJ, van Noort M, Strous GJ, Clevers HC. Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin. EMBO Rep. 2002;3:63–8.
Zhang YK, Huang ZJ, Liu S, Liu YP, Song AA, Song XJ. WNT signaling underlies the pathogenesis of neuropathic pain in rodents. J Clin Invest. 2013;123:2268–86.
Vuong LT, Mlodzik M. Wg/Wnt-signaling-induced nuclear translocation of beta-catenin is attenuated by a beta-catenin peptide through its interference with the IFT-A complex. Cell Rep. 2024;43:114362.
Xia X, Qian S, Soriano S, Wu Y, Fletcher AM, Wang XJ, et al. Loss of presenilin 1 is associated with enhanced beta-catenin signaling and skin tumorigenesis. Proc Natl Acad Sci USA. 2001;98:10863–8.
Hao H, Maeda Y, Fukazawa T, Yamatsuji T, Takaoka M, Bao XH, et al. Inhibition of the growth factor MDK/midkine by a novel small molecule compound to treat non-small cell lung cancer. PLoS One. 2013;8:e71093.
Ishida N, Fukazawa T, Maeda Y, Yamatsuji T, Kato K, Matsumoto K, et al. A novel PI3K inhibitor iMDK suppresses non-small cell lung Cancer cooperatively with A MEK inhibitor. Exp Cell Res. 2015;335:197–206.
Masui M, Okui T, Shimo T, Takabatake K, Fukazawa T, Matsumoto K, et al. Novel Midkine Inhibitor iMDK inhibits tumor growth and angiogenesis in oral squamous cell carcinoma. Anticancer Res. 2016;36:2775–81.
Ueno M, Kariya R, Gunya S, Cheevapruk K, Okada S. Midkine inhibitor (iMDK) induces apoptosis of primary effusion lymphoma via G2/M cell cycle arrest. Leuk Res. 2022;116:106826.
Kang HC, Kim IJ, Park HW, Jang SG, Ahn SA, Yoon SN, et al. Regulation of MDK expression in human cancer cells modulates sensitivities to various anticancer drugs: MDK overexpression confers to a multi-drug resistance. Cancer Lett. 2007;247:40–47.
Tang SL, Gao YL, Chen XB. Wnt/beta-catenin up-regulates Midkine expression in glioma cells. Int J Clin Exp Med. 2015;8:12644–9.
Shi Y, Ge J, Li R, Li Y, Lin L. Targeting of Midkine alleviates cardiac hypertrophy via attenuation of oxidative stress and autophagy. Peptides. 2022;153:170800.
Shin DH, Jo JY, Kim SH, Choi M, Han C, Choi BK, et al. Midkine is a potential therapeutic target of tumorigenesis, angiogenesis, and metastasis in non-small cell lung cancer. Cancers (Basel). 2020;12:2402.
Sueyoshi T, Jono H, Shinriki S, Ota K, Ota T, Tasaki M, et al. Therapeutic approaches targeting Midkine suppress tumor growth and lung metastasis in osteosarcoma. Cancer Lett. 2012;316:23–30.
Acknowledgements
We thank Nathan Cheung and Kristina Dalton for their assistance in the experiments. We thank Reid R Townsend and Petra Gilmore at the Washington University Proteomics Core for quantitative proteomics analysis. We also thank the Siteman Cancer Center’s shared research facilities and the Elizabeth & James McDonnell Endowment for DH.
Funding
This study was supported by the Lung Cancer Research Foundation (Vaishali Kapoor) and K22CA234404 (Vaishali Kapoor).
Author information
Authors and Affiliations
Contributions
MS performed the investigation, analysis, and wrote the manuscript. HKS performed the investigation and analysis. DEH reviewed and edited the manuscript. AKS performed the investigation, analysis, wrote, and reviewed the manuscript. VK conceptualized, supervised, and provided funding for the study, performed investigation and analysis, and wrote and edited the manuscript. All the authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Dr. Hallahan is a founder and shareholder of Medical Guidance Systems LLC, which is developing human anti-TIP1 antibodies. Vaishali Kapoor and Abhay Kumar Singh are inventors on pending patents. Other authors state that there are no conflicts of interest. All authors agree to send an individual conflict of interest form if requested by the journal.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saikia, M., Shah, H.K., Hallahan, D.E. et al. Blocking the functional domain of cancer cell surface TIP1 upregulates Midkine via the β-catenin/Wnt signaling pathway. Cancer Gene Ther 32, 785–792 (2025). https://doi.org/10.1038/s41417-025-00922-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41417-025-00922-8