Abstract
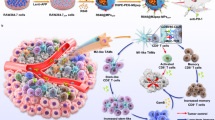
Macrophage infiltration correlates with poor prognosis in patients with liver cancer and resistance to immunotherapy. However, it is difficult to target tumour-associated macrophages (TAMs) because of their inherent heterogeneity. Specific TAM subsets may exhibit distinct functions in tumorigenesis. Herein, we identify a TAM subset characterised by elevated APOE expression, which is correlated with poor overall survival of patients with HCC. The APOE+ TAM intensity is highly elevated in ICB non-responder tumours and negatively correlated with CD8+ T cell infiltration. Pathway analysis and cell interaction reveal that APOE+ TAMs suppress CD8+ T cells through signal integration and cholesterol efflux. Furthermore, APOE deficiency in macrophages delays tumour growth and promotes the infiltration of CD8+ T cells. Using an immunotherapy-resistant mouse model, we showed that APOE blockade synergises with anti-PD-1 therapy and inhibits tumour growth. Our results elucidate the crucial role of APOE+ TAMs in the formation of immunosuppressive microenvironments and offer a potential therapeutic target for ICB combined therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The single-cell RNA sequence datasets used in this study are publicly available in the GEO under accession numbers GSE140228, GSE138709, and GSE206325, and in the China National GeneBank Database (CNGBdb) under accession number CNP0000650. The bulk RNA sequencing is available in GEO under accession number GSE215011.
References
Bray F, Laversanne M, Sung H, Me JF, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2022. https://doi.org/10.3322/caac.21834.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–5.
Sangro B, Gomez-Martin C, De La Mata M, Iñarrairaegui M, Garralda E, Barrera P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–88.
Kambhampati S, Bauer KE, Bracci PM, Keenan BP, Behr SC, Gordan JD, et al. Nivolumab in patients with advanced hepatocellular carcinoma and Child–Pugh class B cirrhosis: safety and clinical outcomes in a retrospective case series. Cancer. 2019;125:3234–41.
Gnjatic S, Bronte V, Brunet LR, Butler MO, Disis ML, Galon J, et al. Identifying baseline immune-related biomarkers to predict clinical outcome of immunotherapy. J Immunother Cancer. 2017;5:44.
Ringelhan M, Pfister D, O’Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222–32.
Zhu G-Q, Tang Z, Huang R, Qu W-F, Fang Y, Yang R, et al. CD36+ cancer-associated fibroblasts provide immunosuppressive microenvironment for hepatocellular carcinoma via secretion of macrophage migration inhibitory factor. Cell Discov. 2023;9:25.
Wang L, Zhu L, Liang C, Huang X, Liu Z, Huo J, et al. Targeting N6-methyladenosine reader YTHDF1 with siRNA boosts antitumor immunity in NASH-HCC by inhibiting EZH2-IL-6 axis. J Hepatol. 2023;79:1185–1200.
Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, et al. Tumor-associated macrophages produce interleukin 6 and sSignal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393–404.
Dhanasekaran R, Venkatesh SK, Torbenson M, Roberts LR. Clinical implications of basic research in hepatocellular carcinoma. J Hepatol. 2017. https://doi.org/10.1016/j.jhep.2015.09.008.
Lai Q, Vitale A, Manzia T, Foschi F, Levi Sandri G, Gambato M, et al. Platelets and hepatocellular cancer: bridging the bench to the clinics. Cancers. 2019;11:1568.
Dong S, Guo X, Han F, He Z, Wang Y. Emerging role of natural products in cancer immunotherapy. Acta Pharm Sin B. 2022;12:1163–85.
Wei C-Y, Zhu M-X, Zhang P-F, Huang X-Y, Wan J-K, Yao X-Z, et al. PKCα/ZFP64/CSF1 axis resets the tumor microenvironment and fuels anti-PD1 resistance in hepatocellular carcinoma. J Hepatol. 2022;77:163–76.
Lu J-C, Zhang P-F, Huang X-Y, Guo X-J, Gao C, Zeng H-Y, et al. Amplification of spatially isolated adenosine pathway by tumor–macrophage interaction induces anti-PD1 resistance in hepatocellular carcinoma. J Hematol Onco. 2021;14:200.
Cheng K, Cai N, Zhu J, Yang X, Liang H, Zhang W. Tumor-associated macrophages in liver cancer: From mechanisms to therapy. Cancer Commun. 2022;42:1112–40.
Liu Y, Xun Z, Ma K, Liang S, Li X, Zhou S, et al. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J Hepatol. 2023;78:770–82.
Tan J, Fan W, Liu T, Zhu B, Liu Y, Wang S, et al. TREM2+ macrophages suppress CD8+ T-cell infiltration after transarterial chemoembolisation in hepatocellular carcinoma. J Hepatol. 2023;79:126–40.
He H, Chen S, Fan Z, Dong Y, Wang Y, Li S, et al. Multi-dimensional single-cell characterization revealed suppressive immune microenvironment in AFP-positive hepatocellular carcinoma. Cell Discov. 2023;9:60.
Lu J-C, Wu L-L, Sun Y-N, Huang X-Y, Gao C, Guo X-J, et al. Macro CD5L+ deteriorates CD8+T cells exhaustion and impairs combination of Gemcitabine-Oxaliplatin-Lenvatinib-anti-PD1 therapy in intrahepatic cholangiocarcinoma. Nat Commun. 2024;15:621.
Li Y, Lu R, Abuduhailili X, Feng Y. Apolipoprotein E: a potential prognostic and diagnostic biomarker for hepatocellular carcinoma. J Hepatocell Carcinoma. 2025;12:301–24.
Wang Y, Zhu G-Q, Yang R, Wang C, Qu W-F, Chu T-H, et al. Deciphering intratumoral heterogeneity of hepatocellular carcinoma with microvascular invasion with radiogenomic analysis. J Transl Med. 2023;21:734.
Wan S, He Q-Y, Yang Y, Liu F, Zhang X, Guo X, et al. SPARC stabilizes ApoE to induce cholesterol-dependent invasion and sorafenib resistance in hepatocellular carcinoma. Cancer Res. 2024;84:1872–88.
Yao J, Wu D, Qiu Y. In vitro analyses of paracrine effects of murine classically activated macrophage on beige adipocyte metabolism. STAR Protoc. 2022;3:101480.
Siegert I, Schatz V, Prechtel AT, Steinkasserer A, Bogdan C, Jantsch J. Electroporation of siRNA into mouse bone marrow-derived macrophages and dendritic cells. In: Li S, Cutrera J, Heller R, Teissie J, editors. Electroporation Protocols. 2014. https://doi.org/10.1007/978-1-4614-9632-8_9.
Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao R, et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell. 2019;179:829–845.e20.
Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–16.
Xiao J, Wang S, Chen L, Ding X, Dang Y, Han M, et al. 25-Hydroxycholesterol regulates lysosome AMP kinase activation and metabolic reprogramming to educate immunosuppressive macrophages. Immunity. 2024;57:1087–1104.e7.
Goossens P, Rodriguez-Vita J, Etzerodt A, Masse M, Rastoin O, Gouirand V, et al. Membrane cholesterol efflux drives tumor-associated macrophage reprogramming and tumor progression. Cell Metab. 2019;29:1376–89.e4.
Li T, Li X, Zamani A, Wang W, Lee C-N, Li M, et al. c-Rel is a myeloid checkpoint for cancer immunotherapy. Nat Cancer. 2020;1:507–17.
Klement JD, Redd PS, Lu C, Merting AD, Poschel DB, Yang D, et al. Tumor PD-L1 engages myeloid PD-1 to suppress type I interferon to impair cytotoxic T lymphocyte recruitment. Cancer Cell. 2023;41:620–36.e9.
Chang Y, Kang P, Cui T, Guo W, Zhang W, Du P, et al. Pharmacological inhibition of demethylzeylasteral on JAK-STAT signaling ameliorates vitiligo. J Transl Med. 2023;21:434.
Sharma A, Seow JJW, Dutertre C-A, Pai R, Blériot C, Mishra A, et al. Onco-fetal reprogramming of endothelial cells drives immunosuppressive macrophages in hepatocellular carcinoma. Cell. 2020;183:377–94.e21.
Li Y, Shen Z, Chai Z, Zhan Y, Zhang Y, Liu Z, et al. Targeting MS4A4A on tumour-associated macrophages restores CD8+ T-cell-mediated antitumour immunity. Gut. 2023;72:2307–20.
Liu C, Xie J, Lin B, Tian W, Wu Y, Xin S, et al. Pan-cancer single-cell and spatial-resolved profiling reveals the immunosuppressive role of APOE+ Macrophages In Immune Checkpoint Inhibitor Therapy. Adv Sci. 2024;11:2401061.
Tharp KM, Kersten K, Maller O, Timblin GA, Stashko C, Canale FP, et al. Tumor-associated macrophages restrict CD8+ T cell function through collagen deposition and metabolic reprogramming of the breast cancer microenvironment. Nat Cancer. 2024;5:1045–62.
Khan MN, Mao B, Hu J, Shi M, Wang S, Rehman AU, et al. Tumor-associated macrophages and CD8+ T cells: dual players in the pathogenesis of HBV-related HCC. Front Immunol. 2024;15:1472430.
Ma S, Sun B, Duan S, Han J, Barr T, Zhang J, et al. YTHDF2 orchestrates tumor-associated macrophage reprogramming and controls antitumor immunity through CD8+ T cells. Nat Immunol. 2023;24:255–66.
Pascual-García M, Bonfill-Teixidor E, Planas-Rigol E, Rubio-Perez C, Iurlaro R, Arias A, et al. LIF regulates CXCL9 in tumor-associated macrophages and prevents CD8+ T cell tumor-infiltration impairing anti-PD1 therapy. Nat Commun. 2019;10:2416.
Franco F, Jaccard A, Romero P, Yu Y-R, Ho P-C. Metabolic and epigenetic regulation of T-cell exhaustion. Nat Metab. 2020;2:1001–12.
Yang R, Sun L, Li C-F, Wang Y-H, Yao J, Li H, et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat Commun. 2021;12:832.
Di Pilato M, Kfuri-Rubens R, Pruessmann JN, Ozga AJ, Messemaker M, Cadilha BL, et al. CXCR6 positions cytotoxic T cells to receive critical survival signals in the tumor microenvironment. Cell. 2021;184:4512–30.e22.
Feig C, Jones JO, Kraman M, Wells RJB, Deonarine A, Chan DS, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti–PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci. 2013;110:20212–7.
Seo YD, Jiang X, Sullivan KM, Jalikis FG, Smythe KS, Abbasi A, et al. Mobilization of CD8+ T cells via CXCR4 blockade facilitates PD-1 checkpoint therapy in human pancreatic cancer. Clin Cancer Res. 2019;25:3934–45.
Gao Y, Shi H, Zhao H, Yao M, He Y, Jiang M, et al. Single-cell transcriptomics identify TNFRSF1B as a novel T-cell exhaustion marker for ovarian cancer. Clin Transl Med. 2023;13:e1416.
Qu Y, Wang X, Bai S, Niu L, Zhao G, Yao Y, et al. The effects of TNF-α/TNFR2 in regulatory T cells on the microenvironment and progression of gastric cancer. Int J Cancer. 2022;15:1373–91.
Rannikko JH, Hollmén M. Clinical landscape of macrophage-reprogramming cancer immunotherapies. Br J Cancer. 2024;131:627–40.
Xiang X, Wang J, Lu D, Xu X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Target Ther. 2021;6:75.
Ling GS, Crawford G, Buang N, Bartok I, Tian K, Thielens NM, et al. C1q restrains autoimmunity and viral infection by regulating CD8 + T cell metabolism. Science. 2018;360:558–63.
Revel M, Sautès-Fridman C, Fridman W-H, Roumenina LT. C1q+ macrophages: passengers or drivers of cancer progression. Trends Cancer. 2022;8:517–26.
Ma R-Y, Black A, Qian B-Z. Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 2022;43:546–63.
Huggins DN, LaRue RS, Wang Y, Knutson TP, Xu Y, Williams JW, et al. Characterizing macrophage diversity in metastasis-bearing lungs reveals a lipid-associated macrophage subset. Cancer Res. 2021;81:5284–95.
Timperi E, Gueguen P, Molgora M, Magagna I, Kieffer Y, Lopez-Lastra S, et al. Lipid-associated macrophages are induced by cancer-associated fibroblasts and mediate immune suppression in breast cancer. Cancer Res. 2022;82:3291–306.
Liu Z, Gao Z, Li B, Li J, Ou Y, Yu X, et al. Lipid-associated macrophages in the tumor-adipose microenvironment facilitate breast cancer progression. OncoImmunology. 2022;11:2085432.
Endo-Umeda K, Makishima M. Liver X receptors regulate cholesterol metabolism and immunity in hepatic nonparenchymal cells. Int J Mol Sci. 2019;20:5045.
King RJ, Singh PK, Mehla K. The cholesterol pathway: impact on immunity and cancer. Trends Immunol. 2022;43:78–92.
Ma X, Bi E, Huang C, Lu Y, Xue G, Guo X, et al. Cholesterol negatively regulates IL-9-producing CD8+ T cell differentiation and antitumor activity. J Exp Med. 2018;215:1555–69.
Ma X. Cholesterol Induces CD8+ T cell exhaustion in the tumor microenvironment. Cell Metab. 2019;30:143–56.e5.
Acknowledgements
We thank Tingbo Liang (The First Affiliated Hospital of Zhejiang University) for providing the Hepa 1–6 cell line.
Funding
This work was supported by grants from the National Natural Science Foundation of China [grant number 82303369 to Yuexiao Tang]; the Natural Science Funding of Zhejiang Province [grant number LQ23H160003 to Yuexiao Tang]; the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission [grant number 2025KY048 to Xiaoxiao Zheng]; the Projects of Lishui Key Research and Development Plan in Zhejiang Province [grant number 2022ZDYF08 to Chaoyong Tu]; the Training Objects of Health Innovative Talents of Zhejiang Health to Hao Liu; the Zhejiang Province Medical Science and Technology Project [grant number 2022ZX00 to Zhiming Hu]; and the State Administration of Traditional Chinese Medicine Science and Technology Department-Zhejiang Provincial Administration of Traditional Chinese Medicine Co-construction of Key Laboratory of Research on Prevention and Treatment for depression syndrome [grant number GZY-ZJ-SY-2402 to Zhiming Hu].
Author information
Authors and Affiliations
Contributions
WC supervised the project and administered the research. YT supervised the project, administered the research, acquired funding, and conducted investigations. TM provided supervision. XX conducted investigations and wrote the original draft. ZZ conducted investigations. XXZ conducted investigations and acquired funding. CT collected human samples, and acquired funding. HL and ZH acquired funding. All authors contributed to the manuscript and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal experiments were performed in accordance with relevant guidelines and regulations and were approved by the Institutional Animal Care and Use Committee of The First Affiliated Hospital, Zhejiang University (Approval Number: 20241278). The research involving human participants was approved by the Ethics Committee of Lishui Central Hospital (Approval Number: 2024-212).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xia, X., Zhou, Z., Zheng, X. et al. APOE deficiency triggers anti-tumour activity of macrophages in liver cancer. Cancer Gene Ther 32, 949–962 (2025). https://doi.org/10.1038/s41417-025-00936-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41417-025-00936-2