Abstract
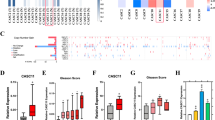
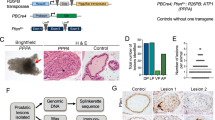
While the functions of activator protein-1 (AP-1) family transcription factors in prostate cancer (PCa) have been well researched, the specific role and mechanisms of FOSB in PCa progression are poorly understood. Here, we aimed to elucidate the precise role of FOSB in PCa and its underlying molecular mechanisms. A comprehensive investigation involving bioinformatics analysis of the TCGA and GEO datasets, validation in clinical PCa samples and cell lines, functional studies in vitro and in vivo, and RNA sequencing coupled with targeted validation (dual-luciferase reporter assays, ChIP‒qPCR, RT‒qPCR, Western blotting, and immunohistochemistry) was performed. FOSB is downregulated in PCa, and its high expression in tumours may reduce the risk of PCa progression by influencing characteristic growth-related cancer pathways. FOSB overexpression significantly inhibited PCa cell proliferation, increased apoptosis in vitro, and attenuated tumour growth in vivo, whereas FOSB knockdown resulted in the opposite effects. Mechanistically, FOSB transcripts were enriched in cell nuclei, where they upregulated the expression of IGFBP5, a gene that modulates the cellular response to IGF-1. This FOSB-mediated upregulation of IGFBP5 expression subsequently weakened the susceptibility of IGF1R to IGF-1 stimulation and suppressed the downstream PI3K/Akt and Ras/Raf/ERK oncogenic pathways. Our findings identify the novel FOSB–IGFBP5–IGF-1 axis upstream of PI3K/Akt and Ras/Raf/ERK signalling as a key regulator of PCa progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The RNA sequencing data discussed in this publication have been deposited in Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series accession number GSE304134. The single-cell dataset generated in this study is available from GEO under the accession numbers GSE221603 and GSE206962. The public data analysed in this study are available from GEO under the accessions GSE200879 and GSE46602. The TCGA prostate adenocarcinoma (PRAD) dataset was downloaded from UCSC Xena (https://xenabrowser.net/datapages/). All the scripts used for data processing and analysis are available from the corresponding author upon reasonable request.
References
Wasim S, Lee SY, Kim J. Complexities of prostate cancer. Int J Mol Sci. 2022;23:14257.
Chang AJ, Autio KA, Roach M 3rd, Scher HI. High-risk prostate cancer-classification and therapy. Nat Rev Clin Oncol. 2014;11:308–23.
Filho AM, Laversanne M, Ferlay J, Colombet M, Piñeros M, Znaor A, et al. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int J Cancer. 2025;156:1336–46.
Rizzo A, Santoni M, Mollica V, Fiorentino M, Brandi G, Massari F. Microbiota and prostate cancer. Semin Cancer Biol. 2022;86:1058–65.
Kennedy TAC, Ong WL, Quon H, Cheung P, Chu W, Chung H, et al. Stereotactic radiation therapy for localized prostate cancer: 10-year outcomes from three prospective trials. Int J Radiat Oncol Biol Phys. 2025;121:325–30.
Gillessen S, Bossi A, Davis ID, de Bono J, Fizazi K, James ND, et al. Management of patients with advanced prostate cancer. Part I: intermediate-/high-risk and locally advanced disease, biochemical relapse, and side effects of hormonal treatment: Report of the Advanced Prostate Cancer Consensus Conference 2022. Eur Urol. 2023;83:267–93.
Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15:701–11.
Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–86.
Mulholland DJ, Tran LM, Li Y, Cai H, Morim A, Wang S, et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell. 2011;19:792–804.
Butler DE, Marlein C, Walker HF, Frame FM, Mann VM, Simms MS, et al. Inhibition of the PI3K/AKT/mTOR pathway activates autophagy and compensatory Ras/Raf/MEK/ERK signalling in prostate cancer. Oncotarget. 2017;8:56698–713.
Ravi P, Wang V, Fichorova RN, McGregor B, Wei XX, Basaria S, et al. IGF-1 axis changes with ADT and docetaxel in metastatic prostate cancer. Endocr Relat Cancer. 2023;30:e230241.
Matsushita M, Fujita K, Hayashi T, Kayama H, Motooka D, Hase H, et al. Gut microbiota-derived short-chain fatty acids promote prostate cancer growth via IGF1 signaling. Cancer Res. 2021;81:4014–26.
Zhao Y, Cai C, Zhang M, Shi L, Wang J, Zhang H, et al. Ephrin-A2 promotes prostate cancer metastasis by enhancing angiogenesis and promoting EMT. J Cancer Res Clin Oncol. 2021;147:2013–23.
Su W, Han HH, Wang Y, Zhang B, Zhou B, Cheng Y, et al. The polycomb repressor complex 1 drives double-negative prostate cancer metastasis by coordinating stemness and immune suppression. Cancer Cell. 2019;36:139–55.e10.
Li N, Liu Q, Han Y, Pei S, Cheng B, Xu J, et al. ARID1A loss induces polymorphonuclear myeloid-derived suppressor cell chemotaxis and promotes prostate cancer progression. Nat Commun. 2022;13:7281.
Yamada Y, Beltran H. The treatment landscape of metastatic prostate cancer. Cancer Lett. 2021;519:20–9.
Guo C, Figueiredo I, Gurel B, Neeb A, Seed G, Crespo M, et al. B7-H3 as a therapeutic target in advanced prostate cancer. Eur Urol. 2023;83:224–38.
Will M, Qin AC, Toy W, Yao Z, Rodrik-Outmezguine V, Schneider C, et al. Rapid induction of apoptosis by PI3K inhibitors is dependent upon their transient inhibition of RAS-ERK signaling. Cancer Discov. 2014;4:334–47.
Siech C, Rutz J, Maxeiner S, Grein T, Sonnenburg M, Tsaur I, et al. Insulin-like growth factor-1 influences prostate cancer cell growth and invasion through an integrin à3, à5, àV, and á1 dependent mechanism. Cancers. 2022;14:363.
Lubik AA, Gunter JH, Hendy SC, Locke JA, Adomat HH, Thompson V, et al. Insulin increases de novo steroidogenesis in prostate cancer cells. Cancer Res. 2011;71:5754–64.
Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3:859–68.
Guo XF, Zhang Z, Zheng L, Zhou YM, Wu HY, Liang CQ, et al. Developmental expression patterns of fosl genes in Xenopus tropicalis. Gene Expr Patterns. 2019;34:119056.
Huo L, Rothstein TL. Receptor-specific induction of individual AP-1 components in B lymphocytes. J Immunol. 1995;154:3300–9.
Fittall MW, Mifsud W, Pillay N, Ye H, Strobl AC, Verfaillie A, et al. Recurrent rearrangements of FOS and FOSB define osteoblastoma. Nat Commun. 2018;9:2150.
Zhang H, Zhang G, Xiao M, Cui S, Jin C, Yang J, et al. Two-polarized roles of transcription factor FOSB in lung cancer progression and prognosis: dependent on p53 status. J Exp Clin Cancer Res. 2024;43:237.
Zhao Y, Tang H, Kuai Y, Xu J, Sun B, Li Y. Identification of the function of FOSB in cholangiocarcinoma using bioinformatics analysis. Transl Cancer Res. 2023;12:3629–40.
Wang S, Yu L, Sun X, Zhang B. Establishment and verification of potential biomarkers for cholangiocarcinoma. Exp Ther Med. 2022;24:546.
Yang C, Chen Y, Tang G, Shen T, Li L. Dysregulation of c-Jun (JUN) and FBJ murine osteosarcoma viral oncogene homolog B (FOSB) in obese people and their predictive values for metabolic syndrome. Endocr J. 2024;71:1157–63.
Skrypnik K, Suliburska J, Skrypnik D, Pilarski Ł, Reguła J, Bogdański P. The genetic basis of obesity complications. Acta Sci Pol Technol Aliment. 2017;16:83–91.
Vivian J, Rao AA, Nothaft FA, Ketchum C, Armstrong J, Novak A, et al. Toil enables reproducible, open source, big biomedical data analyses. Nat Biotechnol. 2017;35:314–6.
Ma X, Zheng J, He K, Wang L, Wang Z, Wang K, et al. TGFA expression is associated with poor prognosis and promotes the development of cervical cancer. J Cell Mol Med. 2024;28:e18086.
Davis S, Meltzer PS. GEOquery: a bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics. 2007;23:1846–7.
Song H, Tian X, He L, Liu D, Li J, Mei Z, et al. CREG1 deficiency impaired myoblast differentiation and skeletal muscle regeneration. J Cachexia Sarcopenia Muscle. 2024;15:587–602.
He K, Li J, Huang X, Zhao W, Wang K, Wang T, et al. KNL1 is a prognostic and diagnostic biomarker related to immune infiltration in patients with uterine corpus endometrial carcinoma. Front Oncol. 2023;13:1090779.
Duan S, Huang W, Liu X, Liu X, Chen N, Xu Q, et al. IMPDH2 promotes colorectal cancer progression through activation of the PI3K/AKT/mTOR and PI3K/AKT/FOXO1 signaling pathways. J Exp Clin Cancer Res. 2018;37:304.
Dong B, Miao J, Wang Y, Luo W, Ji Z, Lai H, et al. Single-cell analysis supports a luminal-neuroendocrine transdifferentiation in human prostate cancer. Commun Biol. 2020;3:778.
Guo W, Li L, He J, Liu Z, Han M, Li F, et al. Single-cell transcriptomics identifies a distinct luminal progenitor cell type in distal prostate invagination tips. Nat Genet. 2020;52:908–18.
He MX, Cuoco MS, Crowdis J, Bosma-Moody A, Zhang Z, Bi K, et al. Transcriptional mediators of treatment resistance in lethal prostate cancer. Nat Med. 2021;27:426–33.
Henry GH, Malewska A, Joseph DB, Malladi VS, Lee J, Torrealba J, et al. A cellular anatomy of the normal adult human prostate and prostatic urethra. Cell Rep. 2018;25:3530–.e5.
Karthaus WR, Hofree M, Choi D, Linton EL, Turkekul M, Bejnood A, et al. Regenerative potential of prostate luminal cells revealed by single-cell analysis. Science. 2020;368:497–505.
Maynard A, McCoach CE, Rotow JK, Harris L, Haderk F, Kerr DL, et al. Therapy-induced evolution of human lung cancer revealed by single-cell RNA sequencing. Cell. 2020;182:1232–.e22.
Cai W, Ma Y, Song L, Cao N, Gao J, Zhou S, et al. IGF-1R down regulates the sensitivity of hepatocellular carcinoma to sorafenib through the PI3K / akt and RAS / raf / ERK signaling pathways. BMC Cancer. 2023;23:87.
Yang H, Li TW, Peng J, Mato JM, Lu SC. Insulin-like growth factor 1 activates methionine adenosyltransferase 2A transcription by multiple pathways in human colon cancer cells. Biochem J. 2011;436:507–16.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Destefanis F, Manara V, Bellosta P. Myc as a regulator of ribosome biogenesis and cell competition: a link to cancer. Int J Mol Sci. 2020;21:4037.
van Riggelen J, Yetil A, Felsher DW. MYC as a regulator of ribosome biogenesis and protein synthesis. Nat Rev Cancer. 2010;10:301–9.
Guo J, Li N, Liu Q, Hao Z, Zhu G, Wang X, et al. KMT2C deficiency drives transdifferentiation of double-negative prostate cancer and confer resistance to AR-targeted therapy. Cancer Cell. 2025;43:1261–78.e10.
Fornes O, Castro-Mondragon JA, Khan A, van der Lee R, Zhang X, Richmond PA, et al. JASPAR 2020: update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2020;48:D87–d92.
McQueeney K, Dealy CN. Roles of insulin-like growth factor-I (IGF-I) and IGF-I binding protein-2 (IGFBP2) and -5 (IGFBP5) in developing chick limbs. Growth Horm IGF Res. 2001;11:346–63.
Salih DA, Tripathi G, Holding C, Szestak TA, Gonzalez MI, Carter EJ, et al. Insulin-like growth factor-binding protein 5 (Igfbp5) compromises survival, growth, muscle development, and fertility in mice. Proc Natl Acad Sci USA. 2004;101:4314–9.
Hwang JR, Cho YJ, Lee Y, Park Y, Han HD, Ahn HJ, et al. The C-terminus of IGFBP-5 suppresses tumor growth by inhibiting angiogenesis. Sci Rep. 2016;6:39334.
Xue X, Li Z, Zhao J, Zhao Z, Li Z, Li Y, et al. Advances in the relationship between AP-1 and tumorigenesis, development and therapy resistance. Discov Oncol. 2025;16:61.
Song D, Lian Y, Zhang L. The potential of activator protein 1 (AP-1) in cancer targeted therapy. Front Immunol. 2023;14:1224892.
Shi D, Mu S, Hu B, Zhang S, Liu J, Zhang Z, et al. Prognostic role of c-Jun activation domain-binding protein-1 in cancer: A systematic review and meta-analysis. J Cell Mol Med. 2021;25:2750–63.
Tsiambas E, Mastronikolis N, PF P, Kyrodimos E, Chrysovergis A, Papanikolaou V, et al. c-Jun/c-Fos complex in laryngeal squamous cell carcinoma. J buon. 2020;25:618–20.
Gao S, Gang J, Yu M, Xin G, Tan H. Computational analysis for identification of early diagnostic biomarkers and prognostic biomarkers of liver cancer based on GEO and TCGA databases and studies on pathways and biological functions affecting the survival time of liver cancer. BMC Cancer. 2021;21:791.
Hu B, Yu M, Ma X, Sun J, Liu C, Wang C, et al. IFNα potentiates anti-PD-1 efficacy by remodeling glucose metabolism in the hepatocellular carcinoma microenvironment. Cancer Discov. 2022;12:1718–41.
Wang Y, Liu M, Liu X, Guo X. LINC00963-FOSB-mediated transcription activation of UBE3C enhances radioresistance of breast cancer cells by inducing ubiquitination-dependent protein degradation of TP73. J Transl Med. 2023;21:321.
Tang C, Jiang Y, Shao W, Shi W, Gao X, Qin W, et al. Abnormal expression of FOSB correlates with tumor progression and poor survival in patients with gastric cancer. Int J Oncol. 2016;49:1489–96.
Park JA, Na HH, HO Jin, Kim KC. Increased expression of FosB through reactive oxygen species accumulation functions as pro-apoptotic protein in piperlongumine treated MCF7 breast cancer cells. Mol Cells. 2019;42:884–92.
Dittmer J. Biological effects and regulation of IGFBP5 in breast cancer. Front Endocrinol. 2022;13:983793.
Wu K, Zhou M, Wu QX, Yuan SX, Wang DX, Jin JL, et al. The role of IGFBP-5 in mediating the anti-proliferation effect of tetrandrine in human colon cancer cells. Int J Oncol. 2015;46:1205–13.
Wang S, Hong Q, Geng X, Chi K, Cai G, Wu D. Insulin-like growth factor binding protein 5-A probable target of kidney renal papillary renal cell carcinoma. Biomed Res Int. 2019;2019:3210324.
Sinha KM, Bagheri-Yarmand R, Lahiri S, Lu Y, Zhang M, Amra S, et al. Oncogenic and osteolytic functions of histone demethylase NO66 in castration-resistant prostate cancer. Oncogene. 2019;38:5038–49.
Chen X, Yu Q, Pan H, Li P, Wang X, Fu S. Overexpression of IGFBP5 enhances radiosensitivity through PI3K-AKT pathway in prostate cancer. Cancer Manag Res. 2020;12:5409–18.
Abdul Hafizz AMH, Mohd Mokthar N, Md Zin RR, N PM, Mamat Yusof MN, Kampan NC, et al. Insulin-like growth factor 1 (IGF1) and its isoforms: insights into the mechanisms of endometrial cancer. Cancers. 2025;17:129.
Liu Y, Shen S, Yan Z, Yan L, Ding H, Wang A, et al. Expression characteristics and their functional role of IGFBP gene family in pan-cancer. BMC Cancer. 2023;23:371.
Yu H. Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–89.
Wang X, Wang Y, Lei P, Qu X, Qi R, Chen D, et al. IGFBP5 regulates fibrocartilage differentiation and cartilage injury induced by T-2 toxin via blocking IGF-1/IGF-1R signalling. Rheumatology. 2025;64:4051–60.
Fan Y, Wu YJ, Guo K, Zhou XQ, Abulaiti A, Olatunji OJ, et al. Interaction with IGF1 overrides ANXA2-mediated anti-inflammatory functions of IGFBP5 in vivo. Front Immunol. 2024;15:1539317.
Pakradooni R, Shukla N, Gupta K, Kumar J, Isali I, Khalifa AO, et al. Diosmetin induces modulation of Igf-1 and Il-6 levels to alter rictor-Akt-PKCà cascade in inhibition of prostate cancer. J Clin Med. 2021;10:4741.
Bertrand FE, Steelman LS, Chappell WH, Abrams SL, Shelton JG, White ER, et al. Synergy between an IGF-1R antibody and Raf/MEK/ERK and PI3K/Akt/mTOR pathway inhibitors in suppressing IGF-1R-mediated growth in hematopoietic cells. Leukemia. 2006;20:1254–60.
Hu L, Shi W, Liu K, Ma D, Xin Q, Wang Z, et al. EGFR bypass activation mediates acquired resistance to regorafenib in hepatocellular carcinoma. Front Med. 2024;11:1464610.
Qian X, Bi QY, Wang ZN, Han F, Liu LM, Song LB, et al. Qingyihuaji Formula promotes apoptosis and autophagy through inhibition of MAPK/ERK and PI3K/Akt/mTOR signaling pathway on pancreatic cancer in vivo and in vitro. J Ethnopharmacol. 2023;307:116198.
Kang JB, Koh PO. Retinoic acid alleviates the reduction of Akt and Bad phosphorylation and regulates Bcl-2 family protein interactions in animal models of ischemic stroke. PLoS ONE. 2024;19:e0303213.
Guefack MF, Talukdar D, Mukherjee R, Guha S, Mitra D, Saha D, et al. Hypericum roeperianum bark extract suppresses breast cancer proliferation via induction of apoptosis, downregulation of PI3K/Akt/mTOR signaling cascade and reversal of EMT. J Ethnopharmacol. 2024;319:117093.
Acknowledgements
The authors would like to acknowledge Guangzhou Science and Technology Plan Project, Guangzhou Health Technology Project, Panyu District Science and Technology Plan Project for their support.
Funding
This work was supported by the National Key Research and Development Program of China (No. 2023YFE0204500), and the National Natural Science Foundation of China (No. 82272856).
Author information
Authors and Affiliations
Contributions
SCZ, QingL, and TW conceived and designed the experiments. JH performed the experiments. JH, SDG, LLZ, and XLS acquired and analysed the data. JH wrote this manuscript. QuL, XFL, and WJT checked the manuscript. All the authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study adhered to the guidelines for animal and human research ethics. The animal experiments in this study were reviewed and approved by the Animal Ethical and Welfare Committee (AEWC) (Approval No. IAEC-K-230615-04). Human study protocols were approved by the Jiangmen Central Hospital Ethics Committee (Approval No. [2023]51), Jiangmen, China. Written informed consent, detailing the study’s objectives, was obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, J., Ge, SD., Zhao, LL. et al. The FOSB-IGFBP5-IGF-1 axis: a novel regulatory pathway that suppresses prostate cancer growth. Cancer Gene Ther (2026). https://doi.org/10.1038/s41417-026-01012-z
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41417-026-01012-z