Abstract
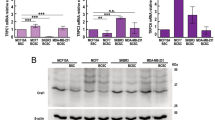
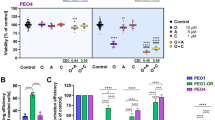
Orai proteins are highly selective calcium channels playing an important role in calcium entry. Orai3 channels are overexpressed in breast cancer (BC) tissues, and involved in their proliferation, cell cycle progression and survival. Herein, we sought to address the involvement of Orai3 in resistance to chemotherapeutic drugs. Using high-throughput approaches, we investigated major changes induced by Orai3 overexpression, including downstream signaling mechanisms involved in BC chemotherapy resistance. Resistance was dependent on external calcium presence and thus Orai3 functionality. This effect allowed a downregulation of the p53 tumor suppressor protein expression via the pro-survival PI3K/Sgk-1/Sek-1 pathway. We demonstrated that p53 degradation occurred not only via Mdm2, but also via another unexpected E3 ubiquitin ligase, Nedd4-2. We found supporting bioinformatic evidence linking Orai3 overexpression and chemoresistance in large human BC data sets. Altogether, our results shed light on the molecular mechanisms activated in BC cells commonly found to overexpress Orai3, allowing resistance to chemotherapeutic drugs.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22.
Li S, Kennedy M, Payne S, Kennedy K, Seewaldt VL, Pizzo SV, et al. Model of tumor dormancy/recurrence after short-term chemotherapy. PLoS ONE. 2014;9:e98021.
Prevarskaya N, Skryma R, Shuba Y. Ion channels and the hallmarks of cancer. Trends Mol Med. 2010;16:107–21.
D’Amico M, Gasparoli L, Arcangeli A. Potassium channels: novel emerging biomarkers and targets for therapy in cancer. Recent Pat Anticancer Drug Discov. 2013;8:53–65.
Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21.
Monteith GR, McAndrew D, Faddy HM, Roberts-Thomson SJ. Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer. 2007;7:519–30.
Berridge MJ, Bootman MD, Lipp P. Calcium - a life and death signal. Nature. 1998;395:645–8.
Putney JW. Capacitative calcium entry: from concept to molecules. Immunol Rev. 2009;231:10–22.
Flourakis M, Lehen’kyi V, Beck B, Raphael M, Vandenberghe M, Abeele FV, et al. Orai1 contributes to the establishment of an apoptosis-resistant phenotype in prostate cancer cells. Cell Death Dis. 2010;1:e75.
Abeele FV, Skryma R, Shuba Y, Van Coppenolle F, Slomianny C, Roudbaraki M, et al. Bcl-2-dependent modulation of Ca2+ homeostasis and store-operated channels in prostate cancer cells. Cancer Cell. 2002;1:169–79.
Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–65.
Shuttleworth TJ. Orai3—the ‘exceptional’ Orai? J Physiol. 2012;590:241–57.
Schindl R, Bergsmann J, Frischauf I, Derler I, Fahrner M, Muik M, et al. 2-Aminoethoxydiphenyl borate alters selectivity of Orai3 channels by increasing their pore size. J Biol Chem. 2008;283:20261–7.
Faouzi M, Hague F, Potier M, Ahidouch A, Sevestre H, Ouadid-Ahidouch H, et al. Down-regulation of Orai3 arrests cell-cycle progression and induces apoptosis in breast cancer cells but not in normal breast epithelial cells. J Cell Physiol. 2011;226:542–51.
Faouzi M, Kischel P, Hague F, Ahidouch A, Benzerdjeb N, Sevestre H, et al. ORAI3 silencing alters cell proliferation and cell cycle progression via c-myc pathway in breast cancer cells. Biochim Biophys Acta. 2013;1833:752–60.
Motiani RK, Abdullaev IF, Trebak M. A novel native store-operated calcium channel encoded by Orai3: selective requirement of Orai3 versus Orai1 in estrogen receptor-positive versus estrogen receptor-negative breast cancer cells. J Biol Chem. 2010;285:19173–83.
Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Zoller M, Fleig A, et al. CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr Biol. 2007;17:794–800.
Oltval ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell. 1993;74:609–19.
Basu A, Haldar S. The relationship between BcI2, Bax and p53: consequences for cell cycle progression and cell death. Mol Hum Reprod. 1998;4:1099–109.
Mooney LM, Al-Sakkaf KA, Brown BL, Dobson PRM. Apoptotic mechanisms in T47D and MCF-7 human breast cancer cells. Br J Cancer. 2002;87:909–17.
Toledo F. Role of TP53 mutations in cancer (an overview). In: Hayat MA, editor. General methods and overviews, lung carcinoma and prostate carcinoma, vol. 2. Netherlands: Springer; 2008. p. 75–92.
Lukashchuk N, Vousden KH, Ubiquitination and degradation of mutant p53. Mol Cell Biol. 2007;27:8284–95
Brickley DR, Agyeman AS, Kopp RF, Hall BA, Harbeck MC, Belova L, et al. Serum- and glucocorticoid-induced protein kinase 1 (SGK1) is regulated by store-operated Ca2+ entry and mediates cytoprotection against necrotic cell death. J Biol Chem. 2013;288:32708–19.
Kobayashi T, Cohen P. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J. 1999;339:319–28.
Sherk AB, Frigo DE, Schnackenberg CG, Bray JD, Laping NJ, Trizna W, et al. Development of a small-molecule serum- and glucocorticoid-regulated kinase-1 antagonist and its evaluation as a prostate cancer therapeutic. Cancer Res. 2008;68:7475–83.
Amato R, D’Antona L, Porciatti G, Agosti V, Menniti M, Rinaldo C, et al. Sgk1 activates MDM2-dependent p53 degradation and affects cell proliferation, survival, and differentiation. J Mol Med. 2009;87:1221–39.
Bhalla V, Daidié D, Li H, Pao AC, LaGrange LP, Wang J, et al. Serum- and glucocorticoid-regulated kinase 1 regulates ubiquitin ligase neural precursor cell-expressed, developmentally down-regulated protein 4-2 by inducing interaction with 14-3-3. Mol Endocrinol. 2005;19:3073–84.
Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84.
Prevarskaya N, Skryma R, Shuba Y. Ca2+ homeostasis in apoptotic resistance of prostate cancer cells. Biochem Biophys Res Commun. 2004;322:1326–35.
Dubois C, Vanden Abeele F, Lehen’kyi V, Gkika D, Guarmit B, Lepage G, et al. Remodeling of channel-forming ORAI proteins determines an oncogenic switch in prostate cancer. Cancer Cell. 2014; 26:19-32.
Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–88.
Giorgi C, Bonora M, Sorrentino G, Missiroli S, Poletti F, Suski JM, et al. p53 at the endoplasmic reticulum regulates apoptosis in a Ca2+-dependent manner. Proc Natl Acad Sci USA. 2015;112:1779–84.
Wu Y, Mehew JW, Heckman CA, Arcinas M, Boxer LM. Negative regulation of bcl-2 expression by p53 in hematopoietic cells. Oncogene. 2001;20:240–51.
Burris H III. Overcoming acquired resistance to anticancer therapy: focus on the PI3K/AKT/mTOR pathway. Cancer Chemother Pharmacol. 2013;71:829–42.
Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16:21–32.
Sommer EM, Dry H, Cross D, Guichard S, Davies BR, Alessi DR, et al. Elevated SGK1 predicts resistance of breast cancer cells to Akt inhibitors. Biochem J. 2013;452:499–508.
Zhao Y, Yu H, Hu W. The regulation of MDM2 oncogene and its impact on human cancers. Acta Biochim Biophys Sin (Shanghai). 2014;46:180–9.
Zheng T, Wang J, Zhao Y, Zhang C, Lin M, Wang X, et al. Spliced MDM2 isoforms promote mutant p53 accumulation and gain-of-function in tumorigenesis. Nat Commun. 2013;4:2996
Wang J, Peng Q, Lin Q, Childress C, Carey D, Yang W, et al. Calcium activates Nedd4 E3 ubiquitin ligases by releasing the C2 domain-mediated auto-inhibition. J Biol Chem. 2010;285:12279–88.
Snyder PM, Olson DR, Thomas BC. Serum and glucocorticoid-regulated kinase modulates Nedd4-2-mediated inhibition of the epithelial Na+ channel. J Biol Chem. 2002;277:5–8.
Chandran S, Li H, Dong W, Krasinska K, Adams C, Alexandrova L, et al. Neural precursor cell-expressed developmentally down-regulated protein 4-2 (Nedd4-2) regulation by 14-3-3 protein binding at canonical serum and glucocorticoid kinase 1 (SGK1) phosphorylation sites. J Biol Chem. 2011;286:37830–40.
Wiemuth D, Lott JS, Ly K, Ke Y, Teesdale-Spittle P, Snyder PM, et al. Interaction of serum- and glucocorticoid regulated kinase 1 (SGK1) with the WW-domains of Nedd4-2 is required for epithelial sodium channel regulation. PLoS ONE. 2010;5:e12163.
Snyder PM, Down-regulating destruction: phosphorylation regulates the E3 ubiquitin ligase Nedd4-2. Sci Signal. 2009;2:pe41
Kim MJ, Chae JS, Kim KJ, Hwang SG, Yoon KW, Kim EK, et al. Negative regulation of SEK1 signaling by serum‐ and glucocorticoid‐inducible protein kinase 1. EMBO J. 2007;26:3075–85.
Iqbal S, Howard S, LoGrasso PV. Serum- and glucocorticoid-inducible kinase 1 confers protection in cell-based and in in vivo neurotoxin models via the c-Jun N-terminal kinase signaling pathway. Mol Cell Biol. 2015;35:1992–2006.
Hawkes WC, Alkan Z. Delayed cell cycle progression in selenoprotein W-depleted cells is regulated by a mitogen-activated protein kinase kinase 4-p38/c-Jun NH2-terminal kinase-p53 pathway. J Biol Chem. 2012;287:27371–9.
Borowiec AS, Hague F, Harir N, Guenin S, Guerineau F, Gouilleux F, et al. IGF-1 activates hEAG K(+) channels through an Akt-dependent signaling pathway in breast cancer cells: role in cell proliferation. J Cell Physiol. 2007;212:690–701.
Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45.
Borowiec A-S, Bidaux G, Tacine R, Dubar P, Pigat N, Delcourt P, et al. Are Orai1 and Orai3 channels more important than calcium influx for cell proliferation? Biochim Biophys Acta. 2014;1843:464–72.
Revet I, Huizenga G, Chan A, Koster J, Volckmann R, van Sluis P, et al. The MSX1 homeobox transcription factor is a downstream target of PHOX2B and activates the Delta-Notch pathway in neuroblastoma. Exp Cell Res. 2008;314:707–19.
Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12:320–7.
Tabchy A, Valero V, Vidaurre T, Lluch A, Gomez H, Martin M, et al. Evaluation of a 30-gene paclitaxel, fluorouracil, doxorubicin, and cyclophosphamide chemotherapy response predictor in a multicenter randomized trial in breast cancer. Clin Cancer Res. 2010;16:5351–61.
Acknowledgements
This study was supported by Région Picardie, le Ministère de l’Education Nationale (France), and la Ligue Nationale Contre le Cancer (SEPTENTRION).
Author information
Authors and Affiliations
Contributions
H.O.-A., P.K., D.G., F.H., D.T., and J.H. designed the experiments. J.H., P.K., D.G., L.R.-D., F.H., and C.L. performed and analyzed the experiments. All authors discussed the results and commented on the manuscript. P.K., J.H., D.G., and H.O.-A. wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Hasna, J., Hague, F., Rodat-Despoix, L. et al. Orai3 calcium channel and resistance to chemotherapy in breast cancer cells: the p53 connection. Cell Death Differ 25, 693–707 (2018). https://doi.org/10.1038/s41418-017-0007-1
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41418-017-0007-1
This article is cited by
-
Combined dandelion extract and all-trans retinoic acid induces cytotoxicity in human breast cancer cells
Scientific Reports (2023)
-
Connecting Calcium-Based Nanomaterials and Cancer: From Diagnosis to Therapy
Nano-Micro Letters (2022)
-
A joint analysis using exome and transcriptome data identifies candidate polymorphisms and genes involved with umbilical hernia in pigs
BMC Genomics (2021)
-
Cardamonin protects against lipopolysaccharide-induced myocardial contractile dysfunction in mice through Nrf2-regulated mechanism
Acta Pharmacologica Sinica (2021)
-
Arachidonic acid-regulated calcium signaling in T cells from patients with rheumatoid arthritis promotes synovial inflammation
Nature Communications (2021)