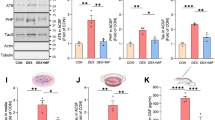
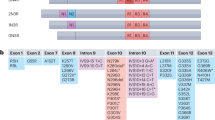
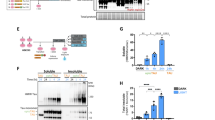
Abstract
Imbalance of neuronal proteostasis associated with misfolding and aggregation of Tau protein is a common neurodegenerative feature in Alzheimer’s disease (AD) and other Tauopathies. Consistent with suggestions that lifetime stress may be an important AD precipitating factor, we previously reported that environmental stress and high glucocorticoid (GC) levels induce accumulation of aggregated Tau; however, the molecular mechanisms for such process remain unclear. Herein, we monitor a novel interplay between RNA-binding proteins (RBPs) and autophagic machinery in the underlying mechanisms through which chronic stress and high GC levels impact on Tau proteostasis precipitating Tau aggregation. Using molecular, pharmacological and behavioral analysis, we demonstrate that chronic stress and high GC trigger mTOR-dependent inhibition of autophagy, leading to accumulation of Tau aggregates and cell death in P301L-Tau expressing mice and cells. In parallel, we found that environmental stress and GC disturb cellular homeostasis and trigger the insoluble accumulation of different RBPs, such as PABP, G3BP1, TIA-1, and FUS, shown to form stress granules (SGs) and Tau aggregation. Interestingly, an mTOR-driven pharmacological stimulation of autophagy attenuates the GC-driven accumulation of Tau and SG-related proteins as well as the related cell death, suggesting a critical interface between autophagy and the response of the SG-related protein in the neurodegenerative potential of chronic stress and GC. These studies provide novel insights into the RNA–protein intracellular signaling regulating the precipitating role of environmental stress and GC on Tau-driven brain pathology.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Sotiropoulos I, Catania C, Pinto LG, Silva R, Pollerberg GE, Takashima A, et al. Stress acts cumulatively to precipitate Alzheimer’s disease-like Tau pathology and cognitive deficits. J Neurosci. 2011;31:7840–7.
Sotiropoulos I, Cerqueira J, Catania C, Takashima A, Sousa N, Almeida O. Stress and glucocorticoid footprints in the brain—the path from depression to Alzheimer’s disease. Neurosci Biobehav Rev. 2008;32:1161–73.
Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, Amaducci LA, et al. Rates and risk factors for dementia and Alzheimer’s disease: results from EURODEM pooled analyses. Neurology. 1999;52:78–84.
Hatzinger M, Z’Brun A, Hemmeter U, Seifritz E, Baumann F, Holsboer-Trachsler E, et al. Hypothalamic–pituitary–adrenal system function in patients with Alzheimer’s disease. Neurobiol Aging. 1995;16:205–9.
Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, et al. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiatry. 2006;163:2164–9.
Simard M, Hudon C, van Reekum R. Psychological distress and risk for dementia. Curr Psychiatry Rep. 2009;11:41–7.
Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and Tau pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2006;26:9047–56.
Sotiropoulos I, Silva J, Kimura T, Rodrigues AJ, Costa P, Almeida OFX, et al. Female hippocampus vulnerability to environmental stress as precipitating factor in Tau aggregation pathology. J Alzheimers Dis. 2014;40:1–12.
Sotiropoulos I, Catania C, Riedemann T, Fry J, Breen K, Michaelidis T, et al. Glucocorticoids trigger Alzheimer disease-like pathobiochemistry in rat neuronal cells expressing human Tau. J Neurochem. 2008;107:385–97.
Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, et al. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci. 2008;28:6926–37.
Nassif M, Hetz C. Autophagy impairment: a crossroad between neurodegeneration and Tauopathies. BMC Biol. 2012;10:78.
Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010;22:132–9.
Caccamo A, Magri A, Medina DX, Wisely EV, Lopez-Aranda MF, Silva AJ, et al. mTOR regulates Tau phosphorylation and degradation: implications for Alzheimer’s disease and other Tauopathies. Aging Cell. 2013;12:370–80.
Vidal RL, Matus S, Bargsted L, Hetz C. Targeting autophagy in neurodegenerative diseases. Trends Pharmacol Sci. 2014;35:583–91.
Seguin SJ, Morelli FF, Vinet J, Amore D, De Biasi S, Poletti A, et al. Inhibition of autophagy, lysosome and VCP function impairs stress granule assembly. Cell Death Differ. 2014;21:1838–51.
Wolozin B. Regulated protein aggregation: stress granules and neurodegeneration. Mol Neurodegener. 2012;7:56.
Apicco DJ, Ash PEA, Maziuk B, Leblang C, Medalla M, Al Abdullatif A, et al. Reducing the RNA binding protein TIA1 protects against Tau-mediated neurodegeneration in vivo. Nat Neurosci. 2018;21:72–82.
Vanderweyde T, Yu H, Varnum M, Liu-Yesucevitz L, Citro A, Ikezu T, et al. Contrasting pathology of the stress granule proteins TIA-1 and G3BP in Tauopathies. J Neurosci. 2012;32:8270–83.
Vanderweyde T, Apicco DJ, Youmans-Kidder K, Ash PEA, Cook C, Lummertz da Rocha E, et al. Interaction of Tau with the RNA-binding protein TIA1 regulates Tau pathophysiology and toxicity. Cell Rep. 2016;15:1455–66.
Kimura T, Sahara N, Yamashita S, Murayama M, Mizoroki T, Yoshiike Y, et al. Aggregation of detergent-insoluble Tau is involved in neuronal loss but not in synaptic loss. J Biol Chem. 2010;285:38692–386999.
Tanemura K, Murayama M, Akagi T, Hashikawa T, Tominaga T, Ichikawa M, et al. Neurodegeneration with Tau accumulation in a transgenic mouse expressing V337M human Tau. J Neurosci. 2002;22:133–41.
Kimura T, Fukuda T, Park JM, Murayama M, Mizoroki T, Yoshiike Y, Sahara N, Takashima AYS. Hyperphosphorylated Tau in parahippocampal cortex impairs place learning in aged mice expressing wild-type human Tau. EMBO J. 2007;26:5143–52.
Sahara N, Lewis J, DeTure M, McGowan E, Dickson DW, Hutton M, et al. Assembly of Tau in transgenic animals expressing P301L Tau: alteration of phosphorylation and solubility. J Neurochem. 2002;83:1498–508.
Bessa JM, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa NFD. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2009;14:764–73.
Sotiropoulos J, Kimura T, Rodirgues AJ, Costa P, ALmeida OFX, Sousa N, Takashima AIS. Female hippocampus vulnerability to environmental stress, a precipitating factor in Tau aggregation pathology. J Alzheimer’s Dis. 2014;40:1–12.
Sun Y, Ip P, Chakrabartty A. Simple elimination of background fluorescence in formalin-fixed human brain tissue for immunofluorescence microscopy. J Vis Exp. 2017. https://doi.org/10.3791/56188.
Jiang T, Yu JT, Zhu XC, Zhang QQ, Cao L, Wang HF, et al. Temsirolimus attenuates tauopathy in vitro and in vivo by targeting Tau hyperphosphorylation and autophagic clearance. Neuropharmacology. 2014;85:121–30.
Korolchuk VI, Menzies FM, Rubinsztein DC. Mechanisms of cross-talk between the ubiquitin-proteasome and autophagy-lysosome systems. FEBS Lett. 2010;584:1393–8.
Giannakopoulos FR, Bussière T, Bouras C, Kövari E, Perl D, Morrison J, et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology. 2003;60:1495–1500.
Wang Y, Mandelkow E. Tau in physiology and pathology. Nat Rev Neurosci. 2015;17:22–35.
Lopes S, Vaz-Silva J, Pinto V, Dalla C, Kokras N, Bedenk B, et al. Tau protein is essential for stress-induced brain pathology. Proc Natl Acad Sci. 2016;113:E3755–E3763.
Pinheiro S, Silva J, Mota C, Vaz-Silva J, Veloso A, Pinto V, et al. Tau mislocation in glucocorticoid-triggered hippocampal pathology. Mol Neurobiol. 2015;53:4745–53.
Jost BC, Grossberg GT. The evolution of psychiatric symptoms in Alzheimer’s disease: a natural history study. J Am Geriatr Soc. 1996;44:1078–81.
Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84.
Wu X-N, Wang X-K, Wu S-Q, Lu J, Zheng M, Wang Y-H, et al. Phosphorylation of Raptor by p38beta participates in arsenite-induced mammalian target of rapamycin complex 1 (mTORC1) activation. J Biol Chem. 2011;286:31501–11.
Corrêa SAL, Eales KL. The role of p38 MAPK and its substrates in neuronal plasticity and neurodegenerative disease. J Signal Transduct. 2012;2012:1–12.
Alam J, Scheper W. Targeting neuronal MAPK14/p38α activity to modulate autophagy in the Alzheimer disease brain. Autophagy. 2016;12:2516–20.
de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–75.
Rissman M, Leea A, Justiceb N, Ricec K, Valed W, Staupa R, et al. Corticotropin-releasing factor receptor-dependent effects of repeated stress on Tau phosphorylation, solubility, and aggregation. Proc Natl Acad Sci. 2012;109:6277–82.
Liu-Yesucevitz L, Bilgutay A, Zhang YJ, Vanderwyde T, Citro A, Mehta T, et al. Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue. PLoS ONE. 2010; 5. https://doi.org/10.1371/journal.pone.0013250.
Lee JY, Koga H, Kawaguchi Y, Tang W, Wong E, Gao YS, et al. HDAC6 controls autophagosome maturation essential for ubiquitin-selective quality-control autophagy. EMBO J. 2010;29:969–80.
Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP. Disease-causing mutations in Parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J Cell Biol. 2010;189:671–9.
Malizzia LJ, Hsu A. Temsirolimus, an mTOR inhibitor for treatment of patients with advanced renal cell carcinoma. Clin J Oncol Nurs. 2008;12:639–46.
Ittner LM, Gotz J. Amyloid-beta and Tau—a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci. 2011;12:65–72.
Soeda Y, Yoshikawa M, Almeida OFX, Sumioka A, Maeda S, Osada H, et al. ARTICLE toxic Tau oligomer formation blocked by capping of cysteine residues with 1,2-dihydroxybenzene groups. Nat Commun. 2015;6:1–12.
Novak P, Schmidt R, Kontsekova E, Zilka N, Kovacech B, Skrabana R, et al. Safety and immunogenicity of the Tau vaccine AADvac1 in patients with Alzheimer’s disease: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol. 2016;4422:123–34.
Brown MR, Bondada V, Keller JN, Thorpe J, Geddes JW. Proteasome or calpain inhibition does not alter cellular Tau levels in neuroblastoma cells or primary neurons. J Alzheimers Dis. 2005;7:15–24.
Feuillette S, Blard O, Lecourtois M, Frebourg T, Campion D, Dumanchin C. Tau is not normally degraded by the proteasome. J Neurosci Res. 2005;80:400–5.
Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–9.
Ambegaokar SS, Jackson GR. The downward spiral of Tau and autolysosomes: a new hypothesis in neurodegeneration. Autophagy. 2012;8:1144–5.
Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285:13107–20.
Li I, Soininen H, Winblad B, Pei JXA. Levels of mTOR and its downstream targets 4E-BP1, eEF2,and eEF2 kinase in relationships with Tau in Alzheimer’s disease brain. FEBS J. 2005;272:4211–20.
Polman JAE, Hunter RG, Speksnijder N, Van Den Oever JME, Korobko OB, McEwen BS, et al. Glucocorticoids modulate the mtor pathway in the hippocampus: differential effects depending on stress history. Endocrinology. 2012;153:4317–27.
An WL, Cowburn RF, Li L, Braak H, Alafuzoff I, Iqbal K, et al. Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer’s disease. Am J Pathol. 2003;163:591–607.
Menzies J, Renna M, Bonin M, Riess O, Rubinsztein DCFMH. Autophagy induction reduces mutant ataxin-3 levels and toxicity in a mouse model of spinocerebellar ataxia type 3. Brain. 2010;133:93–104.
Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol. 1999;147:1431–41.
Gilkes N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, et al. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell. 2004;15:5383–98.
Tourrière H, Chebli K, Zekri L, Courselaud B, Blanchard JM, Bertrand E, et al. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J Cell Biol. 2003;160:823–31.
Maziuk B, Ballance HI, Wolozin B. Dysregulation of RNA binding protein aggregation in neurodegenerative disorders. Front Mol Neurosci. 2017; 10. https://doi.org/10.3389/FNMOL.2017.00089.
Buchan JR, Kolaitis RM, Taylor JP, Parker R XEukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell. 2013; 153. https://doi.org/10.1016/j.cell.2013.05.037.
Pandey UB, Nie Z, Batlevi Y, McCray BA, Ritson GP, Nedelsky NB, et al. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859–63.
Kwon SH. The deacetylase HDAC6 is an essential component of stress granules and plays a critical role in the cellular response to stress. Genes Dev. 2007;21:3381–94.
Seigneurin-Berny D, Verdel A, Curtet S, Lemercier C, Garin J, Rousseaux S, et al. Identification of components of the murine histone deacetylase 6 complex: link between acetylation and ubiquitination signaling pathways. Mol Cell Biol. 2001;21:8035–44.
d’Ydewalle C, Bogaert E, Van Den Bosch L. HDAC6 at the Intersection of neuroprotection and neurodegeneration. Traffic. 2012;13:771–9.
Ding H, Dolan PJ, Johnson GV. Histone deacetylase 6 interacts with the microtubule-associated protein Tau. J Neurochem. 2008;106:2119–30.
Perez M, Santa-Maria I, Gomez de Barreda E, Zhu X, Cuadros R, Cabrero JR, et al. Tau—an inhibitor of deacetylase HDAC6 function. J Neurochem. 2009;109:1756–66.
Chernov KG, Barbet A, Hamon L, Ovchinnikov LP, Curmi PA, Pastr D. Role of microtubules in stress granule assembly: microtubule dynamical instability favors the formation of micrometric stress granules in cells. J Biol Chem. 2009;284:36569–80.
Espallergues J, Teegarden SL, Veerakumar A, Boulden J, Challis C, Jochems J, et al. HDAC6 regulates glucocorticoid receptor signaling in serotonin pathways with critical impact on stress resilience. J Neurosci. 2012;32:4400–16.
Jochems J, Boulden J, Lee BG, Blendy JA, Jarpe M, Mazitschek R, et al. Antidepressant-like properties of novel HDAC6-selective inhibitors with improved brain bioavailability. Neuropsychopharmacology. 2014;39:389–400.
Acknowledgments
We would like to thank Professor Juergen Gotz, (University of Queensland, Australia) for the kind offer of eGFP-P301LTau SH-SY5Y cells and Dr. Bruno Almeida for his technical assistance. J.M.S. was granted with a PhD fellowship (SRFH/BD/88932/2012) by Portuguese Foundation for Science & Technology (FCT); I.S. is holder of FCT Investigator grants (IF/01799/2013), C.D. is a recipient of PhD fellowship of PHDoc program and co-tutelle PhD student of UMinho-UPMC universities. This work was funded by FCT research grants “PTDC/SAU-NMC/113934/2009” (I.S.), the Portuguese North Regional Operational Program (ON.2) under the National Strategic Reference Framework (QREN), through the European Regional Development Fund (FEDER) as well as the Project Estratégico co-funded by FCT (PEst-C/SAU/LA0026/2013) and the European Regional Development Fund COMPETE (FCOMP-01-0124-FEDER-037298) as well as the project NORTE-01-0145-FEDER-000013, supported by the Northern Portugal Regional Operational Program (NORTE 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (FEDER). In addition, this work was partly funded by Canon Foundation in Europe.This work has been also funded by FEDER funds, through the Competitiveness Factors Operational Programme (COMPETE), and by National funds, through the Foundation for Science and Technology (FCT), under the scope of the project POCI-01-0145-FEDER-007038. This study was also supported to BW by grants from NIH (AG050471, NS089544, and ES020395), the BrightFocus Foundation, the Alzheimer Association and the Cure Alzeimer Foundation. Human brain tissue was generously provided by the National Institute of Aging Boston University AD Center (P30AG13846)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
B.W. is co-founder and chief scientific officer of Aquinnah Pharmaceutics Inc. The other authors declare that they have no conflict of interest.
Additional information
Edited by D. Rubinsztein
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Silva, J.M., Rodrigues, S., Sampaio-Marques, B. et al. Dysregulation of autophagy and stress granule-related proteins in stress-driven Tau pathology. Cell Death Differ 26, 1411–1427 (2019). https://doi.org/10.1038/s41418-018-0217-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-018-0217-1
This article is cited by
-
Implications of virus-induced stress granules in tauopathies
Translational Neurodegeneration (2026)
-
Stress granules: emerging players in neurodegenerative diseases
Translational Neurodegeneration (2025)
-
Stress-mediated Activation of Ferroptosis, Pyroptosis, and Apoptosis Following Mild Traumatic Brain Injury Exacerbates Neurological Dysfunctions
Molecular Neurobiology (2025)
-
Fyn-dependent Tau microcluster formation seeds and boosts extensive Tau pathology
Acta Neuropathologica (2025)
-
Cannabidiol-Induced Autophagy Ameliorates Tau Protein Clearance
Neurotoxicity Research (2025)