Abstract
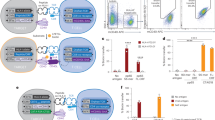
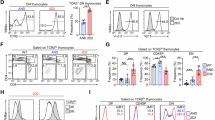
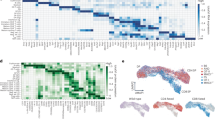
The selection of αβ T cells in the thymus is punctuated by checkpoints at which thymocytes differentiate or undergo apoptosis. Wave 1 deletion is defined as apoptosis within nascent αβ T-cell antigen receptor (TCR)-signalled thymocytes that lack CCR7 expression. The antigen-presenting cell (APC) types that mediate wave 1 deletion are unclear. To measure wave 1 deletion, we compared the frequencies of TCRβ + CD5 + Helios + CCR7– cells in nascent thymocyte cohorts in mice with normal or defective apoptosis. This thymocyte population is small in mice lacking major histocompatibility complex (MHC) expression. The scale of wave 1 deletion was increased by transgenic expression of the self-reactive Yae62 TCRβ chain, was almost halved when haemopoietic APCs lacked MHC expression and, surprisingly, was unchanged when epithelial cells lacked MHC expression. These findings demonstrate efficiency, and some redundancy, in the APC types that mediate wave 1 deletion in the normal mouse thymus.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Daley SR, Teh C, Hu DY, Strasser A, Gray DHD. Cell death and thymic tolerance. Immunol Rev. 2017;277:9–20.
Daley SR, Hu DY, Goodnow CC. Helios marks strongly autoreactive CD4 + T cells in two major waves of thymic deletion distinguished by induction of PD-1 or NF-κB. J Exp Med. 2013;210:269–85.
Hu DY, Yap JY, Wirasinha RC, Howard DR, Goodnow CC, Daley SR. A timeline demarcating two waves of clonal deletion and Foxp3 upregulation during thymocyte development. Immunol Cell Biol. 2016;94:357–66.
Cosgrove D, Chan SH, Waltzinger C, Benoist C, Mathis D. The thymic compartment responsible for positive selection of CD4 + T cells. Int Immunol. 1992;4:707–10.
van Meerwijk JP, Marguerat S, Lees RK, Germain RN, Fowlkes BJ, MacDonald HR. Quantitative impact of thymic clonal deletion on the T cell repertoire. J Exp Med. 1997;185:377–83.
Sinclair C, Bains I, Yates AJ, Seddon B. Asymmetric thymocyte death underlies the CD4:CD8 T-cell ratio in the adaptive immune system. Proc Natl Acad Sci USA. 2013;110:E2905–14.
Stritesky GL, Xing Y, Erickson JR, Kalekar LA, Wang X, Mueller DL, et al. Murine thymic selection quantified using a unique method to capture deleted T cells. Proc Natl Acad Sci USA. 2013;110:4679–84.
Strasser A, Harris AW, von Boehmer H, Cory S. Positive and negative selection of T cells in T-cell receptor transgenic mice expressing a bcl-2 transgene. Proc Natl Acad Sci USA. 1994;91:1376–80.
Stadinski BD, Trenh P, Smith RL, Bautista B, Huseby PG, Li G, et al. A role for differential variable gene pairing in creating T cell receptors specific for unique major histocompatibility ligands. Immunity. 2011;35:694–704.
Penit C, Lucas B, Vasseur F. Cell expansion and growth arrest phases during the transition from precursor (CD4-8-) to immature (CD4 + 8 +) thymocytes in normal and genetically modified mice. J Immunol. 1995;154:5103–13.
Penit C. Localization and phenotype of cycling and post-cycling murine thymocytes studied by simultaneous detection of bromodeoxyuridine and surface antigens. J Histochem Cytochem. 1988;36:473–8.
Lucas B, Vasseur F, Penit C. Normal sequence of phenotypic transitions in one cohort of 5-bromo-2’-deoxyuridine-pulse-labeled thymocytes. Correlation with T cell receptor expression. J Immunol. 1993;151:4574–82.
Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM. Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci USA. 1999;96:14943–8.
Saini M, Sinclair C, Marshall D, Tolaini M, Sakaguchi S, Seddon B. Regulation of Zap70 expression during thymocyte development enables temporal separation of CD4 and CD8 repertoire selection at different signaling thresholds. Sci Signal. 2010;3:ra23.
Mayans S, Stepniak D, Palida SF, Larange A, Dreux J, Arlian BM, et al. T cell receptors expressed by CD4−CD8αβ− intraepithelial T cells drive their fate into a unique lineage with unusual MHC reactivities. Immunity. 2014;41:207–18.
McDonald BD, Bunker JJ, Erickson SA, Oh-Hora M, Bendelac A. Crossreactive αβ T cell receptors are the predominant targets of thymocyte negative selection. Immunity. 2015;43:859–69.
McDonald BD, Bunker JJ, Ishizuka IE, Jabri B, Bendelac A. Elevated T cell receptor signaling identifies a thymic precursor to the TCRαβ+CD4−CD8β− intraepithelial lymphocyte lineage. Immunity. 2014;41:219–29.
Pobezinsky LA, Angelov GS, Tai X, Jeurling S, Van Laethem F, Feigenbaum L, et al. Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nat Immunol. 2012;13:569–78.
Golec DP, Hoeppli RE, Henao Caviedes LM, McCann J, Levings MK, Baldwin TA. Thymic progenitors of TCRαβ+ CD8αα intestinal intraepithelial lymphocytes require RasGRP1 for development. J Exp Med. 2017;214:2421–35.
Ruscher R, Kummer RL, Lee YJ, Jameson SC, Hogquist KA. CD8αα intraepithelial lymphocytes arise from two main thymic precursors. Nat Immunol. 2017;18:771–9.
Wirasinha RC, Singh M, Archer SK, Chan A, Harrison PF, Goodnow CC, et al. T-cell receptors with a central CDR3 cysteine are enriched in CD8αα intraepithelial lymphocytes and their thymic precursors. Immunol Cell Biol. 2018;96:553–61.
Stadinski BD, Shekhar K, Gomez-Tourino I, Jung J, Sasaki K, Sewell AK, et al. Hydrophobic CDR3 residues promote the development of self-reactive T cells. Nat Immunol. 2016;17:946–55.
Chao A, Jost L. Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology. 2012;93:2533–47.
Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK, et al. Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr. 2014;84:45–67.
Guo J, Hawwari A, Li H, Sun Z, Mahanta SK, Littman DR, et al. Regulation of the TCRα repertoire by the survival window of CD4+CD8+ thymocytes. Nat Immunol. 2002;3:469–76.
Perry JS, Lio CW, Kau AL, Nutsch K, Yang Z, Gordon JI, et al. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity. 2014;41:414–26.
McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. J Exp Med. 2008;205:2575–84.
Verstichel G, Vermijlen D, Martens L, Goetgeluk G, Brouwer M, Thiault N, et al. The checkpoint for agonist selection precedes conventional selection in human thymus. Sci Immunol. 2017;2:eaah4232.
Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–89.
Klose CSN, Hummel JF, Faller L, d’Hargues Y, Ebert K, Tanriver Y. A committed postselection precursor to natural TCRαβ+ intraepithelial lymphocytes. Mucosal Immunol. 2018;11:333–44.
Kovalovsky D, Pezzano M, Ortiz BD, Sant’Angelo DB. A novel TCR transgenic model reveals that negative selection involves an immediate, Bim-dependent pathway and a delayed, Bim-independent pathway. PLoS ONE. 2010;5:e8675.
Stepanek O, Prabhakar AS, Osswald C, King CG, Bulek A, Naeher D, et al. Coreceptor scanning by the T cell receptor provides a mechanism for T cell tolerance. Cell. 2014;159:333–45.
Huseby ES, Crawford F, White J, Kappler J, Marrack P. Negative selection imparts peptide specificity to the mature T cell repertoire. Proc Natl Acad Sci USA. 2003;100:11565–70.
Yap JY, Wirasinha RC, Chan A, Howard DR, Goodnow CC, Daley SR. Indirect presentation in the thymus limits naive and regulatory T-cell differentiation by promoting deletion of self-reactive thymocytes. Immunology. 2018;154:522–32.
Koble C, Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J Exp Med. 2009;206:1505–13.
Shugay M, Britanova OV, Merzlyak EM, Turchaninova MA, Mamedov IZ, Tuganbaev TR, et al. Towards error-free profiling of immune repertoires. Nat Methods. 2014;11:653–5.
Bosc N, Lefranc MP. The mouse (Mus musculus) T cell receptor alpha (TRA) and delta (TRD) variable genes. Dev Comp Immunol. 2003;27:465–97.
Acknowledgements
We thank Eric Huseby (University of Massachusetts Medical School) for the gift of the Yae62β-tg and B3K506β-tg mice, Dale Godfrey and Marcin Ciula for the PBS44/CD1d tetramers and the staff of Monash Animal Research Platform, FlowCore and Micromon for technical assistance. This research was supported by the Monash Biomedicine Discovery Institute, by the National Health and Medical Research Council Grant 1107464 to SRD, Grant 1016953 and Australia Fellowship 585490 to CCG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by D.R. Green
Supplementary information
Rights and permissions
About this article
Cite this article
Wirasinha, R.C., Chan, A., Yap, J.Y. et al. Deletion of self-reactive CCR7– thymocytes in the absence of MHC expression on thymic epithelial cells. Cell Death Differ 26, 2727–2739 (2019). https://doi.org/10.1038/s41418-019-0331-8
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41418-019-0331-8
This article is cited by
-
Covalent TCR-peptide-MHC interactions induce T cell activation and redirect T cell fate in the thymus
Nature Communications (2022)
-
Factors that influence the thymic selection of CD8αα intraepithelial lymphocytes
Mucosal Immunology (2021)