Abstract
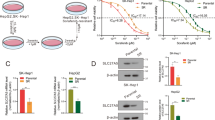
Solute carrier family 27 member 5 (SLC27A5/FATP5) is involved in fatty acid transport and bile acid metabolism; however, little is known about its role in human diseases. Here, we first show that SLC27A5 expression is downregulated in hepatocellular carcinoma (HCC) by DNA hypermethylation, and reduced SCL27A5 expression contributes to tumor progression and poor prognosis. Both gain- and loss-of-function studies demonstrated that SLC27A5 has an antiproliferative effect on HCC cells in vitro and in vivo. Knockout of SLC27A5 increases polyunsaturated lipids, leading to increased NADP+/NADPH ratio, ROS production as well as lipid peroxidation and the subsequent accumulation of 4-hydroxy-2-nonenal (4-HNE) in hepatoma cells. Mass spectrometry analysis found that 4-HNE directly modifies cysteine residues (Cys513, 518) on KEAP1, thus leading KEAP1/NRF2 pathway activation and increases the expression levels of NRF2 target genes, such as TXNRD1. Further, SLC27A5 expression negatively correlates with TXNRD1 expression in hepatoma cells and clinical HCC samples, and blockade of NRF2/TXNRD1 using genetic approaches or inhibitors sensitizes SLC27A5-deficient hepatoma cells to sorafenib treatment. Collectively, we demonstrated that SLC27A5 acts as a novel tumor suppressor by suppressing TXNRD1 expression via the KEAP1/NRF2 pathway in HCC. Combination therapy of sorafenib and NRF2/TXNRD1 inhibitors may be a promising strategy in personalized HCC treatment.
Similar content being viewed by others

Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–64.
Calvisi DF, Wang C, Ho C, Ladu S, Lee SA, Mattu S, et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140:1071–83.e5.
Mashek DG. Hepatic fatty acid trafficking: multiple forks in the road. Adv Nutr Int Rev J. 2013;4:697–710.
Anderson CM, Stahl A. SLC27 fatty acid transport proteins. Mol Asp Med. 2013;34:516–28.
Doege H, Baillie RA, Ortegon AM, Tsang B, Wu Q, Punreddy S, et al. Targeted deletion of FATP5 reveals multiple functions in liver metabolism: alterations in hepatic lipid homeostasis. Gastroenterology. 2006;130:1245–58.
Hubbard B, Doege H, Punreddy S, Wu H, Huang X, Kaushik VK, et al. Mice deleted for fatty acid transport protein 5 have defective bile acid conjugation and are protected from obesity. Gastroenterology. 2006;130:1259–69.
Auinger A, Valenti L, Pfeuffer M, Helwig U, Herrmann J, Fracanzani AL, et al. A promoter polymorphism in the liver-specific fatty acid transport protein 5 is associated with features of the metabolic syndrome and steatosis. Horm Metab Res. 2010;42:854–9.
Panieri E, Santoro M. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis. 2016;7:e2253.
Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12:931–47.
Ray PD, Huang B-W, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–90.
Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radic Biol Med. 2014;66:75–87.
Harris IS, Treloar AE, Inoue S, Sasaki M, Gorrini C, Lee KC, et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell. 2015;27:211–22.
Fu B, Meng W, Zeng X, Zhao H, Liu W, Zhang T. TXNRD1 is an unfavorable prognostic factor for patients with hepatocellular carcinoma. BioMed Res Int. 2017;2017:1–8.
Yoo M-H, Xu X-M, Carlson BA, Gladyshev VN, Hatfield DL. Thioredoxin reductase 1 deficiency reverses tumor phenotype and tumorigenicity of lung carcinoma cells. J Biol Chem. 2006;281:13005–8.
Ungerstedt JS, Sowa Y, Xu W-S, Shao Y, Dokmanovic M, Perez G, et al. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc Natl Acad Sci. 2005;102:673–8.
Duan Y, Tian L, Gao Q, Liang L, Zhang W, Yang Y, et al. Chromatin remodeling gene ARID2 targets cyclin D1 and cyclin E1 to suppress hepatoma cell progression. Oncotarget. 2016;7:45863–75.
Gao Q, Wang K, Chen K, Liang L, Zheng Y, Zhang Y, et al. HBx protein‐mediated ATOH1 downregulation suppresses ARID2 expression and promotes hepatocellular carcinoma. Cancer Sci. 2017;108:1328–37.
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–308.
Long M, Rojo de la Vega M, Wen Q, Bharara M, Jiang T, Zhang R, et al. An essential role of NRF2 in diabetic wound healing. Diabetes. 2016;65:780–93.
Conner DA. Mouse embryo fibroblast (MEF) feeder cell preparation. Curr Protoc Mol Biol. 2000;51:23.2. 1–23.2.7.
Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 2014;14:709–21.
Schönfeld P, Wojtczak L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic Biol Med. 2008;45:231–41.
Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–22.
Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39:199–218.
Levonen AL, Landar A, Ramachandran A, Ceaser EK, Dickinson DA, Zanoni G, et al. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem J. 2004;378:373–82.
McMahon M, Lamont DJ, Beattie KA, Hayes JD. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc Natl Acad Sci. 2010;107:18838–43.
Inami Y, Waguri S, Sakamoto A, Kouno T, Nakada K, Hino O, et al. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J Cell Biol. 2011;193:275–84.
Wang M, Han J, Xing H, Zhang H, Li Z, Liang L, et al. Dysregulated fatty acid metabolism in hepatocellular carcinoma. Hepatic Oncol. 2016;3:241–51.
Nath A, Li I, Roberts LR, Chan C. Elevated free fatty acid uptake via CD36 promotes epithelial-mesenchymal transition in hepatocellular carcinoma. Sci Rep. 2015;5:14752.
Veglia F, Tyurin VA, Blasi M, De Leo A, Kossenkov AV, Donthireddy L, et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature. 2019;569:73–8.
Zhang M, Martino JSD, Bowman RL, Campbell NR, Baksh SC, Simon-Vermot T, et al. Adipocyte-derived lipids mediate melanoma progression via FATP proteins. Cancer Discov. 2018;8:1006–25.
Nath A, Chan C. Genetic alterations in fatty acid transport and metabolism genes are associated with metastatic progression and poor prognosis of human cancers. Sci Rep. 2016;6:18669.
Knapp P, Chabowski A, Harasiuk D, Górski J. Reversed glucose and fatty acids transporter expression in human endometrial cancer. Horm Metab Res. 2012;44:436–41.
Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–62.
Ogretmen B. Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer. 2017;18:33–50.
Lovat PE, Di Sano F, Corazzari M, Fazi B, Donnorso RP, Pearson ADJ, et al. Gangliosides link the acidic sphingomyelinase-mediated induction of ceramide to 12-lipoxygenase-dependent apoptosis of neuroblastoma in response to fenretinide. J Natl Cancer Inst. 2004;96:1288–99.
Li H, Junk P, Huwiler A, Burkhardt C, Wallerath T, Pfeilschifter J, et al. Dual effect of ceramide on human endothelial cells: induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation. 2002;106:2250–6.
Alvaro A, Rosales R, Masana L, Vallvé J-C. Polyunsaturated fatty acids down-regulate in vitro expression of the key intestinal cholesterol absorption protein NPC1L1: no effect of monounsaturated nor saturated fatty acids. J Nutr Biochem. 2010;21:518–25.
Schulze K. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505.
Luo Y, Eggler AL, Liu D, Liu G, Mesecar AD, van Breemen RB. Sites of alkylation of human Keap1 by natural chemoprevention agents. J Am Soc Mass Spectrom. 2007;18:2226–32.
Saito R, Suzuki T, Hiramoto K, Asami S, Naganuma E, Suda H, et al. Characterizations of three major cysteine sensors of Keap1 in stress response. Mol Cell Biol. 2016;36:271–84.
Rojo de la Vega M, Chapman E, Zhang DD. NRF2 and the hallmarks of cancer. Cancer Cell. 2018;34:21–43.
Eriksson SE, Prast-Nielsen S, Flaberg E, Szekely L, Arnér ESJ. High levels of thioredoxin reductase 1 modulate drug-specific cytotoxic efficacy. Free Radic Biol Med. 2009;47:1661–71.
Hu J, Zhang H, Cao M, Wang L, Wu S, Fang B. Auranofin enhances Ibrutinib’s anticancer activity in EGFR-mutant lung adenocarcinoma. Mol Cancer Ther. 2018;17:2156–63.
Acknowledgements
We would like to thank Dr T-C He (University of Chicago, USA) for providing the plasmids pAdEasy system, and Prof. Ding Xue (Tsinghua University) for supplying the CRISPR/Cas9 system. We also thank Prof. Yiguo Zhang (Chongqing University) for providing the pGL3-ARE plasmid. This study was supported by research grants from the China National Natural Science Foundation (grant nos. 81872270 and 81572683 to NT, 81602417 to KW, 81661148057 to AH), the Major National S&T program (2017ZX10202203-004 to NT), Natural Science Foundation Project of CQ CSTC (grant no. cstc2018jcyjAX0254 to NT), the Program for Innovation Team of Higher Education in Chongqing (grant no. CXTDX201601015), the Leading Talent Program of CQ CSTC (grant no. CSTCCXLJRC201719 to NT), the Scientific Research Innovation Project for Postgraduate in Chongqing and Talent Development Program of CQMU for Postgraduate (grant no. BJRC201705).
Author information
Authors and Affiliations
Contributions
AH, NT and KW conceived the study and designed the experiments. QZG, GJZ, YQZ and YY performed most experiments and analyzed the data. CC and JX responded to bioinformatics and statistical analysis. LL collected clinical HCC samples. CL generated KEAP1 mutants. YH and XFC assisted with xenograft assays. WLZ assisted with ROS staining. HT and YXC provided guidance and advice. QZG, NT and KW prepared the manuscript with all authors providing feedback.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by R.A. Knight
Supplementary information
Rights and permissions
About this article
Cite this article
Gao, Q., Zhang, G., Zheng, Y. et al. SLC27A5 deficiency activates NRF2/TXNRD1 pathway by increased lipid peroxidation in HCC. Cell Death Differ 27, 1086–1104 (2020). https://doi.org/10.1038/s41418-019-0399-1
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41418-019-0399-1
This article is cited by
-
Metabolic reprogramming in hepatocellular carcinoma: mechanisms and therapeutic implications
Experimental & Molecular Medicine (2025)
-
Suppression of A-to-I RNA-editing enzyme ADAR1 sensitizes hepatocellular carcinoma cells to oxidative stress through regulating Keap1/Nrf2 pathway
Experimental Hematology & Oncology (2024)
-
The role of ferroptosis in cardio-oncology
Archives of Toxicology (2024)
-
Role of lipid metabolism in hepatocellular carcinoma
Discover Oncology (2024)
-
Identification of potential ferroptosis hub genes in acute-on-chronic liver failure based on bioinformatics analysis and experimental verification
BMC Medical Genomics (2023)