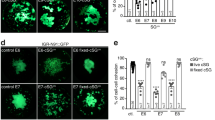
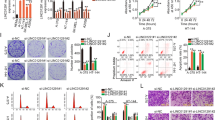
Abstract
The RNA-binding protein LIN28B regulates developmental timing and determines stem cell identity by suppressing the let-7 family of microRNAs. Postembryonic reactivation of LIN28B impairs cell commitment to differentiation, prompting their transformation. In this study, we assessed the extent to which ectopic lin28b expression modulates the physiological behavior of neural crest cells (NCC) and governs their transformation in the trunk region of developing embryos. We provide evidence that the overexpression of lin28b inhibits sympathoadrenal cell differentiation and accelerates NCC migration in two vertebrate models, Xenopus leavis and Danio rerio. Our results highlight the relevance of ITGA5 and ITGA6 in the LIN28B-dependent regulation of the invasive motility of tumor cells. The results also establish that LIN28B overexpression supports neuroblastoma onset and the metastatic potential of malignant cells through let-7a-dependent and let-7a-independent mechanisms.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Change history
18 November 2019
A Correction to this paper has been published: https://doi.org/10.1038/s41418-019-0451-1
References
Kerosuo L, Bronner-Fraser M. What is bad in cancer is good in the embryo: importance of EMT in neural crest development. Semin Cell Dev Biol. 2012;3:320–32.
Anderson DJ, Carnahan JF, Michelsohn A, Patterson PH. Antibody markers identify a common progenitor to sympathetic neurons and chromaffin cells in vivo and reveal the timing of commitment to neuronal differentiation in the sympathoadrenal lineage. J Neurosci. 1991;11:3507–19.
Kulesa PM, Gammill LS. Neural crest migration: patterns, phases and signals. Dev Biol. 2010;2:566–8.
King CE, Wang L, Winograd R, Madison BB, Mongroo PS, Johnstone CN, et al. LIN28B fosters colon cancer migration, invasion and transformation through let-7-dependent and-independent mechanisms. Oncogene. 2011;30:4185–93.
Xiong H, Zhao W, Wang J, Seifer BJ, Ye C, Chen Y, et al. Oncogenic mechanisms of Lin28 in breast cancer: new functions and therapeutic opportunities. Oncotarget. 2017;8:25721–35.
Hamano R, Miyata H, Yamasaki M, Sugimura K, Tanaka K, Kurokawa Y, et al. High expression of Lin28 is associated with tumour aggressiveness and poor prognosis of patients in oesophagus cancer. Br J Cancer. 2012;106:1415–23.
Molenaar JJ, Domingo-Fernández R, Ebus ME, Lindner S, Koster J, Drabek K, et al. LIN28B induces NB and enhances MYCN levels via let-7 suppression. Nat Genet. 2012;44:1199–206.
Luksch R, Castellani MR, Collini P, De Bernardi B, Conte M, Gambini C, et al. NB (Peripheral neuroblastic tumours). Crit Rev Oncol Hematol. 2016;107:163–81.
De Preter K, Vandesompele J, Heimann P, Yigit N, Beckman S, Schramm A, et al. Human fetal neuroblast and NB transcriptome analysis confirms neuroblast origin and highlights NB candidate genes. Genome Biol. 2006;7:R84.
Shimada H, Ambros IM, Dehner LP, Hata JI, Joshi VV, Roald B, et al. The International NB Pathology Classification (the Shimada system). Cancer. 1999;86:364–72.
Cohn SL, Pearson ADJ, London WB, Monclair T, Ambros PF, Brodeur GM, et al. The International NB Risk Group (INRG) classification system: an INRG task force report. J Clin Oncol. 2009;27:289–97.
Pugh TJ, Morozova O, Attiyeh EF, Asgharzadeh S, Wei JS, Auclair D, et al. The genetic landscape of high-risk NB. Nat Genet. 2013;45:279–84.
Moss EG, Lee RC, Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell. 1997;88:637–46.
Tsialikas J, Romer-Seibert J. LIN28: roles and regulation in development and beyond. Development. 2015;142:2397–404.
Zhou J, Ng SB, Chng WJ. LIN28/LIN28B: an emerging oncogenic driver in cancer stem cells. Int J Biochem Cell Biol. 2013;5:973–8.
Helsmoortel HH, Bresolin S, Lammens T, Cavé H, Noellke P, Caye A, et al. LIN28B overexpression defines a novel fetal-like subgroup of juvenile myelomonocytic leukemia. Blood. 2016;127:1163–72.
Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–76.
Naef V, Monticelli S, Corsinovi D, Mazzetto MT, Cellerino A, Ori M. The age-regulated zinc finger factor ZNF367 is a new modulator of neuroblast proliferation during embryonic neurogenesis. Sci Rep. 2018;8:11836.
Stewart RA, Look AT, Kanki JP, Henion PD. Development of the peripheral sympathetic nervous system in zebrafish. Methods Cell Biol. 2004;76:237–60.
Teitelman G, Baker H, Joh TH, Reis DJ. Appearance of catecholamine-synthesizing enzymes during development of rat sympathetic nervous system: Possible role of tissue environment. Proc Natl Acad Sci USA. 1979;76:509–13.
Dutton JR, Antonellis A, Carney TJ, Rodrigues FSLM, Pavan WJ, Ward A, et al. An evolutionarily conserved intronic region controls the spatiotemporal expression of the transcription factor Sox10. BMC Dev Biol. 2008;8:105.
Luo R, An M, Arduini BL, Henion PD. Specific pan-neural crest expression of zebrafish crestin throughout embryonic development. Dev Dyn. 2001;220:169–74.
Richards M. The transcriptome profile of human embryonic stem cells as defined by SAGE. Stem Cells. 2004;22:51–64.
Kelsh RN, Brand M, Jiang YJ, Heisenberg CP, Lin S, Haffter P, et al. Zebrafish pigmentation mutations and the processes of neural crest development. Development. 1996;123:369–89.
Theveneau E, Mayor R. Neural crest delamination and migration: from epithelium-to-mesenchyme transition to collective cell migration. Dev Biol. 2012;336:34–54.
Heanue TA, Shepherd IT, Burns AJ. Enteric nervous system development in avian and zebrafish models. Dev Biol. 2016;417:129–38.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20.
Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;3:371–79.
Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100.
Kragtorp KA, Miller JR. Integrin alpha5 is required for somite rotation and boundary formation in Xenopus. Dev Dyn. 2007;236:2713–20.
Julich D, Cobb G, Melo AM, McMillen P, Lawton AK, Mochrie SG, et al. Cross-scale integrin regulation organizes ECM and tissue topology. Dev Cell. 2015;34:33–44.
Banning A, Babuke T, Kurrle N, Meister M, Ruonala M, Tikkanen R. Flotillins regulate focal adhesions by interacting with a-actinin and by influencing the activation of focal adhesion kinase. Cells. 2018;7:E28.
Owens DW, McLean GW, Wyke AW, Paraskeva C, Parkinson EK, Frame MC, et al. The catalytic activity of the Src family kinases is required to disrupt cadherin-dependent cell–cell contacts. Mol Biol Cell. 2000;11:51–64.
Avizienyte E, Fincham VJ, Brunton VG, Frame MG. Src SH3/2 domain-mediated peripheral accumulation of Src and phospho-myosin is linked to deregulation of E-cadherin and the epithelial–mesenchymal transition. Mol Biol Cell. 2004;15:2794–803.
Wang H, Zhao Q, Deng K, Guo X, Xia J. Lin28: an emerging important oncogene connecting several aspects of cancer. Tumour Biol. 2016;37:2841–8.
Permuth-Wey J, Kim D, Tsai YY, Lin HY, Chen YA, Barnholtz-Sloan J, et al. LIN28B polymorphisms influence susceptibility to epithelial ovarian cancer. Cancer Res. 2011;71:3896–903.
Nguyen LH, Robinton DA, Seligson MT, Wu L, Li L, Rakheja D, et al. LIN28B is sufficient to drive liver cancer and necessary for its maintenance in murine models. Cancer Cell. 2014;26:248–61.
Zhu S, Lee JS, Guo F, Shin J, Perez-Atayde AR, Kutok JL, et al. Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer Cell. 2012;21:362–73.
Hennchen M, Stubbusch J, Abarchan-El Makhfi I, Kramer M, Deller T, Pierre-Eugene C, et al. LIN28B and Let-7 in the control of sympathetic neurogenesis and NB development. J Neurosci. 2015;35:16531–44.
Kalappurakkal JM, Anilkumarr AA, Patra C, van Zanten TS, Sheetz MP, Mayor S. Integrin mechano-chemical signaling generates plasma membrane nanodomains that promote cell spreading. Cell. 2019;177:1738–56.
Laudato S, Patil N, Abba ML, Leupold JH, Benner A, Gaiser T, et al. P53-induced miR-30e-5p inhibits colorectal cancer invasion and metastasis by targeting ITGA6 and ITGB1. Int J Cancer. 2017;141:1879–90.
Brooks DLP, Schwab LP, Krutilina R, Parke DN, Sethuraman A, Hoogewijs D, et al. ITGA6 is directly regulated by hypoxia-inducible factors and enriches for cancer stem cell activity and invasion in metastatic breast cancer models. Mol Cancer. 2016;15:26.
Zhu S, Zhang X, Weichert-Leahey N, Dong Z, Zhang C, Lopez G, et al. LMO1 synergizes with MYCN to promote NB initiation and metastasis. Cancer Cell. 2017;32:310–23.
Kim TH, Kim HI, Soung YH, Shaw LA, Chung J. Integrin (alpha6beta4) signals through Src to increase expression of S100A4, a metastasis promoting factor: implications for cancer cell invasion. Mol Cancer Res. 2009;7:1605–12.
Avizienyte E, Frame MC. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr Opin Cell Biol. 2005;17:542–7.
Crawford BD, Henry CA, Clason TA, Becker AL, Hille MB. Activity and distribution of paxillin, focal adhesion kinase, and cadherin indicate cooperative roles during zebrafish morphogenesis. Mol Biol Cell. 2003;14:3065–81.
Bilozur ME, Hay ED. Neural crest migration in 3D extracellular matrix utilizes laminin, fibronectin, or collagen. Dev Biol. 1988;125:19–33.
Westerfield M. The zebrafish book. a guide for the laboratory use of zebrafish (Danio rerio). 3rd ed. Eugene, OR: Univ Oregon Press; 1995.
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310.
Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, et al. The Tol2kit: a multisite gateway-based construction Kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236:3088–99.
Holzschuh J, Barrallo-Gimeno A, Ettl AK, Durr K, Knapik EW, Driever W. Noradrenergic neurons in the zebrafish hindbrain are induced by retinoic acid and require tfap2a for expression of the neurotransmitter phenotype. Development. 2003;130:5741–54.
Allende ML, Weinberg ES. The expression pattern of two zebrafish achaete-scute homolog (ash) genes is altered in the embryonic brain of the cyclops mutant. Dev Biol. 1994;166:509–30.
Etard C, Gradl D, Kunz M, Eilers M, Wedlich D. Pontin and Reptin regulate cell proliferation in early Xenopus embryos in collaboration with c-Myc and Miz-1. Mech Dev. 2005;122:545–56.
Giannetti K, Corsinovi D, Rossino C, Appolloni I, Malatesta P, Ori M. Platelet derived growth factor B gene expression in the Xenopus laevis developing central nervous system. Int J Dev Biol. 2016;60:175–9.
Thisse B, Thisse C. In situ hybridization on whole-mount zebrafish embryos and young larvae. Methods Mol Biol. 2014;1211:53–67.
Corallo D, Schiavinato A, Trapani V, Moro E, Argenton F, Bonaldo P. Emilin3 is required for notochord sheath integrity and interacts with Scube2 to regulate notochord-derived Hedgehog signals. Development. 2013;140:4594–601.
Aveic S, Corallo D, Porcù E, Pantile M, Boso D, Zanon C, et al. TP-0903 inhibits neuroblastoma cell growth and enhances the sensitivity to conventional chemotherapy. Eur J Pharm. 2018;818:435–48.
Acknowledgements
The authors would like to thank the Fondazione Italiana per la Lotta al Neuroblastoma and the Fondazione Veronesi (FUV) for their support. We also thank Elisa Lidron (University of Padua), Valentina Tonelotto (University of Padua), and Claudia Grigoletto (University of Trieste) for their excellent technical assistance.
Funding
This work was supported by funds from Fondazione Italiana per la Lotta al Neuroblastoma and from the Fondazione Istituto di Ricerca Pediatrica Città della Speranza (project number 19_07IRP). Corallo Diana was supported by a Fondazione Veronesi (FUV) Postdoctoral fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by E. Baehrecke
Supplementary information
Rights and permissions
About this article
Cite this article
Corallo, D., Donadon, M., Pantile, M. et al. LIN28B increases neural crest cell migration and leads to transformation of trunk sympathoadrenal precursors. Cell Death Differ 27, 1225–1242 (2020). https://doi.org/10.1038/s41418-019-0425-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-019-0425-3
This article is cited by
-
Genetic predisposition and chromosome instability in neuroblastoma
Cancer and Metastasis Reviews (2020)