Abstract
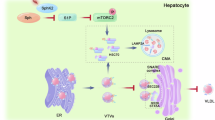
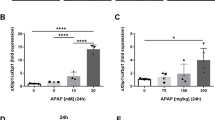
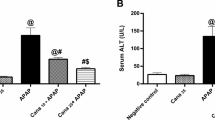
Acetaminophen (APAP) is the leading cause of drug-induced acute liver failure. Sphingosine-1-phosphate (S1P), whose formation is catalyzed by sphingosine kinase (SPHK)-1 or -2, is a bioactive lipid implicated in human health and disease. Here, we show that APAP-treated sphK1-deficient (sphK1−/−) mice exhibited markedly less liver damage and reduced inflammation compared with the wild-type mice. SPHK1 deficiency alleviated APAP-induced endoplasmic reticulum (ER) stress by affecting the phosphorylation of inositol-requiring enzyme 1α (IRE1α) and protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK)-eukaryotic translation initiation factor 2α (eIF2α), levels of activating transcription factor 4 (ATF4), and activation of activating transcription factor 6 (ATF6). SPHK1 deficiency also inhibited mitochondrial permeability transition (MPT), as evidenced by the impaired phosphorylation of JNK, apoptosis signal-regulated kinase 1 (ASK1), and glycogen synthase kinase 3β (GSK3β). In addition, SPHK1 deficiency reduced the levels of histone deacetylase and promoted the acetylation of p65 and STAT1, thereby impairing the transcription of inflammatory genes. Supplementation with exogenous S1P significantly reversed the activation of the PERK-eIF2α-ATF4 pathway and ATF6 during ER stress as well as the activation of GSK3β, ASK1, and JNK during MPT. Both FTY720, a functional S1P receptor antagonist, and PF543, an SPHK1 inhibitor, significantly ameliorated APAP-induced liver injury and improved animal survival. Our study reveals a critical role for SPHK1 in mediating APAP-induced hepatotoxicity by promoting ER stress and MPT.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54.
Schilling A, Corey R, Leonard M, Eghtesad B. Acetaminophen: old drug, new warnings. Clevel Clin J Med. 2010;77:19–27.
Lee WM. Acetaminophen toxicity: changing perceptions on a social/medical issue. Hepatology. 2007;46:966–70.
Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci USA. 1984;81:1327–31.
Coles B, Wilson I, Wardman P, Hinson JA, Nelson SD, Ketterer B. The spontaneous and enzymatic reaction of N-acetyl-p-benzoquinonimine with glutathione: a stopped-flow kinetic study. Arch Biochem Biophys. 1988;264:253–60.
Hinson JA, Pohl LR, Monks TJ, Gillette JR. Acetaminophen-induced hepatotoxicity. Life Sci. 1981;29:107–16.
Lawson JA, Farhood A, Hopper RD, Bajt ML, Jaeschke H. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol Sci. 2000;54:509–16.
Williams CD, Bajt ML, Farhood A, Jaeschke H. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int. 2010;30:1280–92.
Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300–12.
Uzi D, Barda L, Scaiewicz V, Mills M, Mueller T, Gonzalez-Rodriguez A, et al. CHOP is a critical regulator of acetaminophen-induced hepatotoxicity. J Hepatol. 2013;59:495–503.
Dara L, Ji C, Kaplowitz N. The contribution of endoplasmic reticulum stress to liver diseases. Hepatology. 2011;53:1752–63.
Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–62.
Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529:326–35.
Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795–809.
Lisbona F, Rojas-Rivera D, Thielen P, Zamorano S, Todd D, Martinon F, et al. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell. 2009;33:679–91.
Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6.
Rothe M, Sarma V, Dixit VM, Goeddel DV. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995;269:1424–7.
Seki E, Brenner DA, Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143:307–20.
Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–77.
Han D, Dara L, Win S, Than TA, Yuan L, Abbasi SQ, et al. Regulation of drug-induced liver injury by signal transduction pathways: critical role of mitochondria. Trends Pharm Sci. 2013;34:243–53.
Win S, Than TA, Zhang J, Oo C, Min RWM, Kaplowitz N. New insights into the role and mechanism of c-Jun-N-terminal kinase signaling in the pathobiology of liver diseases. Hepatology. 2018;67:2013–24.
Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, et al. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–21.
Chang CH, Randolph GJ. Sphingosine-1-phosphate as the lymphocyte's ticket to ride and survive. Dev Cell. 2017;41:576–8.
Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–63.
Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22:50–60.
Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–15.
Liu H, Ma Y, He HW, Zhao WL, Shao RG. SPHK1 (sphingosine kinase 1) induces epithelial-mesenchymal transition by promoting the autophagy-linked lysosomal degradation of CDH1/E-cadherin in hepatoma cells. Autophagy. 2017;13:900–13.
Yang L, Yue S, Yang L, Liu X, Han Z, Zhang Y, et al. Sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis is involved in liver fibrosis- associated angiogenesis. J Hepatol. 2013;59:114–23.
Win S, Than TA, Fernandez-Checa JC, Kaplowitz N. JNK interaction with Sab mediates ER stress induced inhibition of mitochondrial respiration and cell death. Cell Death Dis. 2014;5:e989.
Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, et al. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–99.
Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–8.
Vienken H, Mabrouki N, Grabau K, Claas RF, Rudowski A, Schomel N, et al. Characterization of cholesterol homeostasis in sphingosine-1-phosphate lyase-deficient fibroblasts reveals a Niemann-Pick disease type C-like phenotype with enhanced lysosomal Ca(2+) storage. Sci Rep. 2017;7:43575.
Zhang H, Li W, Sun S, Yu S, Zhang M, Zou F. Inhibition of sphingosine kinase 1 suppresses proliferation of glioma cells under hypoxia by attenuating activity of extracellular signal-regulated kinase. Cell Prolif. 2012;45:167–75.
Lee SY, Hong IK, Kim BR, Shim SM, Sung Lee J, Lee HY, et al. Activation of sphingosine kinase 2 by endoplasmic reticulum stress ameliorates hepatic steatosis and insulin resistance in mice. Hepatology. 2015;62:135–46.
Zhu X, Shi D, Cao K, Ru D, Ren J, Rao Z, et al. Sphingosine kinase 2 cooperating with Fyn promotes kidney fibroblast activation and fibrosis via STAT3 and AKT. Biochim Biophys Acta Mol Basis Dis. 2018;1864:3824–36.
Ferrari A, Fiorino E, Longo R, Barilla S, Mitro N, Cermenati G, et al. Attenuation of diet-induced obesity and induction of white fat browning with a chemical inhibitor of histone deacetylases. Int J Obes. 2017;41:289–98.
Lorenzo Pisarello M, Masyuk TV, Gradilone SA, Masyuk AI, Ding JF, Lee PY, et al. Combination of a histone deacetylase 6 inhibitor and a somatostatin receptor agonist synergistically reduces hepatorenal cystogenesis in an animal model of polycystic liver disease. Am J Pathol. 2018;188:981–94.
Chen S, Ye J, Chen X, Shi J, Wu W, Lin W, et al. Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-kappaB pathway dependent of HDAC3. J Neuroinflammation. 2018;15:150.
Kumar P, Gogulamudi VR, Periasamy R, Raghavaraju G, Subramanian U, Pandey KN. Inhibition of HDAC enhances STAT acetylation, blocks NF-kappaB, and suppresses the renal inflammation and fibrosis in Npr1 haplotype male mice. Am J Physiol. 2017;313:F781–F795.
Kopec AK, Joshi N, Cline-Fedewa H, Wojcicki AV, Ray JL, Sullivan BP, et al. Fibrin(ogen) drives repair after acetaminophen-induced liver injury via leukocyte alphaMbeta2 integrin-dependent upregulation of Mmp12. J Hepatol. 2017;66:787–97.
Price VF, Jollow DJ. Effect of glucose and gluconeogenic substrates on fasting-induced suppression of acetaminophen glucuronidation in the rat. Biochem Pharm. 1989;38:289–97.
Acknowledgements
This work was supported by grants from the National Key Research and Development Program of China (Grant No. 2016YFF0101400), National Natural Science Foundation of China (Grant No. 81571614, 81871241), and Jiangsu Provincial Innovation Team Program Foundation.
Author information
Authors and Affiliations
Contributions
LjL and HZ conceptualized and designed the study; LjL, HW, JZ, YS, and FW performed the experiments; LjL performed the statistical analysis; LjL, HW, and HZ drafted the manuscript; SW, LS, QY, MS, LxL, and HZ revised and approved the final version of the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by G. Del Sal
Rights and permissions
About this article
Cite this article
Li, L., Wang, H., Zhang, J. et al. SPHK1 deficiency protects mice from acetaminophen-induced ER stress and mitochondrial permeability transition. Cell Death Differ 27, 1924–1937 (2020). https://doi.org/10.1038/s41418-019-0471-x
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41418-019-0471-x
This article is cited by
-
Sphingosine kinase 2 deficiency impairs VLDL secretion by inhibiting mTORC2 phosphorylation and activating chaperone-mediated autophagy
Cell Death & Differentiation (2025)
-
Advances in drug-induced liver injury research: in vitro models, mechanisms, omics and gene modulation techniques
Cell & Bioscience (2024)
-
CXCL4 deficiency limits M4 macrophage infiltration and attenuates hyperoxia-induced lung injury
Molecular Medicine (2024)
-
Selectively targeting the AdipoR2-CaM-CaMKII-NOS3 axis by SCM-198 as a rapid-acting therapy for advanced acute liver failure
Nature Communications (2024)
-
Triggering of endoplasmic reticulum stress via ATF4-SPHK1 signaling promotes glioblastoma invasion and chemoresistance
Cell Death & Disease (2024)