Abstract
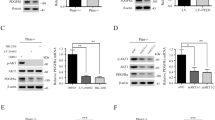
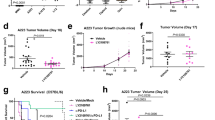
Lack of insight into the identity of the cells that initiate metastasis hampers the development of antimetastatic therapies. Only a tiny fraction of tumor cells termed metastasis-initiating cells (MICs) are able to successfully seed metastases, causing recurrence and therapeutic resistance. Using metastasis models, we describe a subpopulation of MIC derivates from lung metastases that do not have proliferation advantages, express high levels of the PDGF receptors and EMT/stemness-related genes, and are unique in their ability to initiate metastasis. PDGF factors specifically boost the metastatic potential of MIC populations in a PDGFR-dependent manner. However, PDGFR inhibition preferentially suppresses lung metastases, but does not reduce the primary tumor burden. Thus, we found that PDGFR inhibition blocks AKT activation, whereas SGK1, which shares high-similarity kinase domain and overlap substrates with AKT overexpression remains active in MICs. SGK1 and PDGF signaling act in concert to promote metastatic formation, and SGK1 inhibition confers vulnerability to PDGFR inhibitors, also eliciting a powerful antitumor effect. In vivo, SGK1 inhibitors sensitize xenograft tumors to PDGFR-targeted therapies by reducing primary tumor growth and lung metastasis. Consequently, dual inhibition of PDGFR and SGK1 exhibited strong antitumor activities in established breast cancer cell lines in vitro and in vivo. Therefore, this approach not only provides insight into MIC transformation but also aids the design of improved therapeutic strategies for advanced breast cancer.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Spano D, Heck C, De Antonellis P, Christofori G, Zollo M. Molecular networks that regulate cancer metastasis. Semin Cancer Biol. 2012;22:234–49.
Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68.
Cameron MD, Schmidt EE, Kerkvliet N, Nadkarni KV, Morris VL, Groom AC, et al. Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res. 2000;60:2541–6.
Celia-Terrassa T, Kang Y. Distinctive properties of metastasis-initiating cells. Genes Dev. 2016;30:892–908.
Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–7.
Jacob LS, Vanharanta S, Obenauf AC, Pirun M, Viale A, Socci ND, et al. Metastatic competence can emerge with selection of preexisting oncogenic alleles without a need of new mutations. Cancer Res. 2015;75:3713–9.
Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–13.
Lawson DA, Bhakta NR, Kessenbrock K, Prummel KD, Yu Y, Takai K, et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526:131–5.
Pascual G, Avgustinova A, Mejetta S, Martin M, Castellanos A, Attolini CS, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–5.
Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–4.
Goel S, Wang Q, Watt AC, Tolaney SM, Dillon DA, Li W, et al. Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors. Cancer Cell. 2016;29:255–69.
Yang L, Tang H, Kong Y, Xie X, Chen J, Song C, et al. LGR5 promotes breast cancer progression and maintains stem-like cells through activation of Wnt/beta-catenin signaling. Stem Cells. 2015;33:2913–24.
Shibue T, Brooks MW, Weinberg RA. An integrin-linked machinery of cytoskeletal regulation that enables experimental tumor initiation and metastatic colonization. Cancer Cell. 2013;24:481–98.
Fais P, Leopizzi M, Di Maio V, Longo L, Della Rocca C, Tagliaro F, et al. Phosphoinositide-specific phospholipase C in normal human liver and in alcohol abuse. J Cell Biochem. 2019;120:7907–17.
Pelzl L, Tolios A, Schmidt EM, Alesutan I, Walker B, Munzer P, et al. Translational regulation of the serum- and glucocorticoid-inducible kinase-1 (SGK1) in platelets. Biochem Biophy Res Commun. 2012;425:1–5.
Orlacchio A, Ranieri M, Brave M, Arciuch VA, Forde T, De Martino D, et al. SGK1 is a critical component of an AKT-independent pathway essential for PI3K-mediated tumor development and maintenance. Cancer Res. 2017;77:6914–26.
Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42(Database issue):D92–7.
Greenawalt EJ, Edmonds MD, Jain N, Adams CM, Mitra R, Eischen CM. Targeting of SGK1 by miR-576-3p Inhibits Lung Adenocarcinoma Migration and Invasion. Mol Cancer Res. 2019;17:289–98.
Liu W, Wang X, Wang Y, Dai Y, Xie Y, Ping Y, et al. SGK1 inhibition-induced autophagy impairs prostate cancer metastasis by reversing EMT. J Exp Clin Cancer Res. 2018;37:73.
Murray JT, Campbell DG, Morrice N, Auld GC, Shpiro N, Marquez R, et al. Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. Biochemical J. 2004;384(Pt 3):477–88.
Sommer EM, Dry H, Cross D, Guichard S, Davies BR, Alessi DR. Elevated SGK1 predicts resistance of breast cancer cells to Akt inhibitors. Biochemical J. 2013;452:499–508.
Gupta GP, Perk J, Acharyya S, de Candia P, Mittal V, Todorova-Manova K, et al. ID genes mediate tumor reinitiation during breast cancer lung metastasis. Proc Natl Acad Sci USA. 2007;104:19506–11.
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–24.
Oshimori N, Oristian D, Fuchs E. TGF-beta promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell. 2015;160:963–76.
Feng XH. The changing faces of cancer cells. Nat Rev Mol cell Biol. 2010;11:466.
Papadopoulos N, Lennartsson J. The PDGF/PDGFR pathway as a drug target. Mol Asp Med. 2018;62:75–88.
Hensel JA, Flaig TW, Theodorescu D. Clinical opportunities and challenges in targeting tumour dormancy. Nat Rev Clin Oncol. 2013;10:41–51.
Castel P, Ellis H, Bago R, Toska E, Razavi P, Carmona FJ, et al. PDK1-SGK1 signaling sustains AKT-independent mTORC1 activation and confers resistance to PI3Kalpha inhibition. Cancer Cell. 2016;30:229–42.
Lang F, Gawaz M, Borst O. The serum- & glucocorticoid-inducible kinase in the regulation of platelet function. Acta Physiol. 2015;213:181–90.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 81672598, 81872152, 81772961, and 81702620).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by H.-U. Simon
Rights and permissions
About this article
Cite this article
Yang, L., Li, N., Xue, Z. et al. Synergistic therapeutic effect of combined PDGFR and SGK1 inhibition in metastasis-initiating cells of breast cancer. Cell Death Differ 27, 2066–2080 (2020). https://doi.org/10.1038/s41418-019-0485-4
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41418-019-0485-4
This article is cited by
-
Targeting CAF-specific metabolic pathways in breast cancer
Naunyn-Schmiedeberg's Archives of Pharmacology (2025)
-
Hepatocyte SGK1 activated by hepatic ischemia-reperfusion promotes the recurrence of liver metastasis via IL-6/STAT3
Journal of Translational Medicine (2023)
-
Induction of SGK1 via glucocorticoid-influenced clinical outcome of triple-negative breast cancer patients
Breast Cancer Research and Treatment (2023)
-
Protein tyrosine kinase inhibitor resistance in malignant tumors: molecular mechanisms and future perspective
Signal Transduction and Targeted Therapy (2022)
-
N6-methyladenosine regulated FGFR4 attenuates ferroptotic cell death in recalcitrant HER2-positive breast cancer
Nature Communications (2022)