Abstract
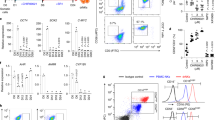
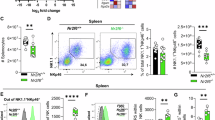
Natural killer (NK) cell development is a multistep process that requires a variety of signals and transcription factors. The lack of mammalian target of rapamycin (mTOR) kinase severely impairs NK cell development in mice. mTOR binds to Raptor and Rictor to form two complexes, mTORC1 and mTORC2, respectively. How mTOR and its two complexes regulate NK cell development is not fully understood. Here, we developed two methods to inactivate mTOR, Raptor, or Rictor in early stage NK cells (using CD122-Cre) or in late-stage NK cells (using Ncr1-CreTg). First, we found that when mTOR was deleted by CD122-Cre during and after NK cell commitment, NK cell development was severely impaired, while Ncr1-CreTg mediated mTOR deletion slightly affected NK cell terminal differentiation, suggesting that mTOR is essential for early NK cell differentiation. Second, we found that CD122-mediated deletion of Raptor significantly limited the differentiation of CD27+CD11b− immature NK (iNK) cell into mature NK cells. In contrast, the absence of Rictor significantly interfered with the differentiation of CD27−CD11b− early iNK cells. Third, Ncr1-mediated deletion of Raptor, rather than Rictor, moderately affected NK cell terminal differentiation. In terms of mechanism, mTORC1 mainly promotes the expression of NK cell-specific transcription factor E4 promoter-binding protein 4 (E4BP4), while both mTORC1 and mTORC2 can enhance the expression of T-bet. Therefore, mTORC1 and mTORC2 subtly coordinate NK cell development by differentially inducing E4BP4 and T-bet.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Narni-Mancinelli E, Chaix J, Fenis A, Kerdiles YM, Yessaad N, Reynders A, et al. Fate mapping analysis of lymphoid cells expressing the NKp46 cell surface receptor. Proc Natl Acad Sci USA. 2011;108:18324–9.
Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–96.
Goh W, Huntington ND. Regulation of murine natural killer cell development. Front Immunol. 2017;8:130.
Male V, Nisoli I, Kostrzewski T, Allan DS, Carlyle JR, Lord GM, et al. The transcription factor E4bp4/Nfil3 controls commitment to the NK lineage and directly regulates Eomes and Id2 expression. J Exp Med. 2014;211:635–42.
Gascoyne DM, Long E, Veiga-Fernandes H, de Boer J, Williams O, Seddon B, et al. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10:1118–24.
Crotta S, Gkioka A, Male V, Duarte JH, Davidson S, Nisoli I, et al. The transcription factor E4BP4 is not required for extramedullary pathways of NK cell development. J Immunol. 2014;192:2677–88.
Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC, et al. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 2012;36:55–67.
Simonetta F, Pradier A, Roosnek E. T-bet and Eomesodermin in NK cell development, maturation, and function. Front Immunol. 2016;7:241.
Daussy C, Faure F, Mayol K, Viel S, Gasteiger G, Charrier E, et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J Exp Med. 2014;211:563–77.
Yang M, Li D, Chang Z, Yang Z, Tian Z, Dong Z. PDK1 orchestrates early NK cell development through induction of E4BP4 expression and maintenance of IL-15 responsiveness. J Exp Med. 2015;212:253–65.
Kovanen PE, Leonard WJ. Cytokines and immunodeficiency diseases: critical roles of the gamma(c)-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol Rev. 2004;202:67–83.
Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–80.
Donnelly RP, Loftus RM, Keating SE, Liou KT, Biron CA, Gardiner CM, et al. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J Immunol. 2014;193:4477–84.
Nandagopal N, Ali AK, Komal AK, Lee SH. The critical role of IL-15-PI3K-mTOR pathway in natural killer cell effector functions. Front Immunol. 2014;5:187.
Marcais A, Cherfils-Vicini J, Viant C, Degouve S, Viel S, Fenis A, et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat Immunol. 2014;15:749–57.
Yang C, Tsaih SW, Lemke A, Flister MJ, Thakar MS, Malarkannan S. mTORC1 and mTORC2 differentially promote natural killer cell development. Elife. 2018;7:e35619.
Wang F, Meng M, Mo B, Yang Y, Ji Y, Huang P, et al. Crosstalks between mTORC1 and mTORC2 variagate cytokine signaling to control NK maturation and effector function. Nat Commun. 2018;9:4874.
Kalim KW, Zhang S, Chen X, Li Y, Yang JQ, Zheng Y, et al. mTOR has a developmental stage-specific role in mitochondrial fitness independent of conventional mTORC1 and mTORC2 and the kinase activity. PLoS ONE. 2017;12:e0183266.
Pimeisl IM, Tanriver Y, Daza RA, Vauti F, Hevner RF, Arnold HH, et al. Generation and characterization of a tamoxifen-inducible Eomes (CreER) mouse line. Genesis. 2013;51:725–33.
Funding
Research reported in this publication was supported by the Natural Science Foundation of China (to ZD, 81725007, 31830027, 31821003, and 91942308; to MY, 81771666, and 81471523; to WX, 31700771), National Key Research & Developmental Program of China (to ZD, 2018YFC1003900), Natural Science Foundation of Guangdong Province (to MY, 2019A1515011707), Guangzhou Science and Technology Project (to MY, 201707010395), and by 111 Project (B16201).
Author information
Authors and Affiliations
Contributions
DL and YW performed and analyzed experiments; DL, MY, and ZD designed experiments, analyzed data, and wrote the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures of animals were approved by the Animal Ethics Committee of Tsinghua University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by G. Melino
Rights and permissions
About this article
Cite this article
Li, D., Wang, Y., Yang, M. et al. mTORC1 and mTORC2 coordinate early NK cell development by differentially inducing E4BP4 and T-bet. Cell Death Differ 28, 1900–1909 (2021). https://doi.org/10.1038/s41418-020-00715-6
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41418-020-00715-6
This article is cited by
-
NK cell immunopotentiators-loaded nanoliposomes enhance ADCC effect for targeted therapy against HER2-positive breast cancer
Cell Communication and Signaling (2025)
-
Generation of mature epicardium derived from human-induced pluripotent stem cells via inhibition of mTOR signaling
Nature Communications (2025)
-
Melatonin enhances NK cell function in aged mice by increasing T-bet expression via the JAK3-STAT5 signaling pathway
Immunity & Ageing (2024)
-
Hippo kinases Mst1 and Mst2 maintain NK cell homeostasis by orchestrating metabolic state and transcriptional activity
Cell Death & Disease (2024)
-
The role of basic leucine zipper transcription factor E4BP4 in cancer: a review and update
Molecular Biology Reports (2024)