Abstract
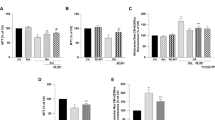
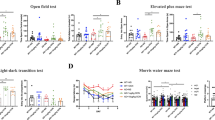
Hyperglycemia in diabetes mellitus (DM) patients is a causative factor for amyloidogenesis and induces neuropathological changes, such as impaired neuronal integrity, neurodegeneration, and cognitive impairment. Regulation of mitochondrial calcium influx from the endoplasmic reticulum (ER) is considered a promising strategy for the prevention of mitochondrial ROS (mtROS) accumulation that occurs in the Alzheimer’s disease (AD)-associated pathogenesis in DM patients. Among the metabolites of ellagitannins that are produced in the gut microbiome, urolithin A has received an increasing amount of attention as a novel candidate with anti-oxidative and neuroprotective effects in AD. Here, we investigated the effect of urolithin A on high glucose-induced amyloidogenesis caused by mitochondrial calcium dysregulation and mtROS accumulation resulting in neuronal degeneration. We also identified the mechanism related to mitochondria-associated ER membrane (MAM) formation. We found that urolithin A-lowered mitochondrial calcium influx significantly alleviated high glucose-induced mtROS accumulation and expression of amyloid beta (Aβ)-producing enzymes, such as amyloid precursor protein (APP) and β-secretase-1 (BACE1), as well as Aβ production. Urolithin A injections in a streptozotocin (STZ)-induced diabetic mouse model alleviated APP and BACE1 expressions, Tau phosphorylation, Aβ deposition, and cognitive impairment. In addition, high glucose stimulated MAM formation and transglutaminase type 2 (TGM2) expression. We first discovered that urolithin A significantly reduced high glucose-induced TGM2 expression. In addition, disruption of the AIP–AhR complex was involved in urolithin A-mediated suppression of high glucose-induced TGM2 expression. Markedly, TGM2 silencing inhibited inositol 1, 4, 5-trisphosphate receptor type 1 (IP3R1)–voltage-dependent anion-selective channel protein 1 (VDAC1) interactions and prevented high glucose-induced mitochondrial calcium influx and mtROS accumulation. We also found that urolithin A or TGM2 silencing prevented Aβ-induced mitochondrial calcium influx, mtROS accumulation, Tau phosphorylation, and cell death in neuronal cells. In conclusion, we suggest that urolithin A is a promising candidate for the development of therapies to prevent DM-associated AD pathogenesis by reducing TGM2-dependent MAM formation and maintaining mitochondrial calcium and ROS homeostasis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Nagai N, Ito Y, Sasaki H. Hyperglycemia enhances the production of amyloid β1-42 in the lenses of otsuka long-evans tokushima fatty rats, a model of human type 2 diabetes. Invest Ophthalmol Vis Sci. 2016;57:1408–17.
Lee HJ, Seo HI, Cha HY, Yang YJ, Kwon SH, Yang SJ. Diabetes and Alzheimer’s disease: mechanisms and nutritional aspects. Clin Nutr Res. 2018;7:229–40.
Yang Y, Wu Y, Zhang S, Song W. High glucose promotes Aβ production by inhibiting APP degradation. PLoS ONE. 2013;8:e69824.
Macauley SL, Stanley M, Caesar EE, Yamada SA, Raichle ME, Perez R, et al. Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo. J Clin Invest. 2015;125:2463–7.
Lee HJ, Ryu JM, Jung YH, Lee SJ, Kim JY, Lee SH, et al. High glucose upregulates BACE1-mediated Aβ production through ROS-dependent HIF-1α and LXRα/ABCA1-regulated lipid raft reorganization in SK-N-MC cells. Sci Rep. 2016;6:36746.
McConlogue L, Buttini M, Anderson JP, Brigham EF, Chen KS, Freedman SB, et al. Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP Transgenic Mice. J Biol Chem. 2007;282:26326–34.
Ohno M, Sametsky EA, Younkin LH, Oakley H, Younkin SG, Citron M, et al. BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer’s disease. Neuron. 2004;41:27–33.
Kumar P, Raman T, Swain MM, Mishra R, Pal A. Hyperglycemia-induced oxidative-nitrosative stress induces inflammation and neurodegeneration via augmented tuberous sclerosis complex-2 (TSC-2) activation in neuronal cells. Mol Neurobiol. 2017;54:238–54.
Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB. Diabetes mellitus and Alzheimer’s disease: shared pathology and treatment? Br J Clin Pharm. 2011;71:365–76.
Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F. Oxidative stress and the amyloid β peptide in Alzheimer’s disease. Redox Biol. 2018;14:450–64.
Onphachanh X, Lee HJ, Lim JR, Jung YH, Kim JS, Chae CW, et al. Enhancement of high glucose-induced PINK1 expression by melatonin stimulates neuronal cell survival: involvement of MT2 /Akt/NF-κB pathway. J Pineal Res. 2017;63:e12427.
Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci. 2008;9:36–45.
Chen W, Yang J, Chen S, Xiang H, Liu H, Lin D, et al. Importance of mitochondrial calcium uniporter in high glucose-induced endothelial cell dysfunction. Diab Vasc Dis Res. 2017;14:494–501.
Liu ZJ, Zhao W, Lei HY, Xu HL, Lai LY, Xu R, et al. High glucose enhances bupivacaine-induced neurotoxicity via MCU-mediated oxidative stress in SH-SY5Y cells. Oxid Med Cell Longev. 2019;2019:7192798.
Vance JE. MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochim Biophys Acta. 2014;1841:595–609.
Pinho CM, Teixeira PF, Glaser E. Mitochondrial import and degradation of amyloid-β peptide. Biochim Biophys Acta. 2014;1837:1069–74.
Jadiya P, Kolmetzky DW, Tomar D, Di Meco A, Lombardi AA, Lambert JP, et al. Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer’s disease. Nat Commun. 2019;10:3885.
Area-Gomez E, Del Carmen Lara Castillo M, Tambini MD, Guardia-Laguarta C, de Groof AJ, Madra M, et al. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 2012;31:4106–23.
Hedskog L, Pinho CM, Filadi R, Ronnback A, Hertwig L, Wiehager B, et al. Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer’s disease and related models. Proc Natl Acad Sci USA. 2013;110:7916–21.
Xu H, Guan N, Ren YL, Wei QJ, Tao YH, Yang GS, et al. IP3R-Grp75-VDAC1-MCU calcium regulation axis antagonists protect podocytes from apoptosis and decrease proteinuria in an Adriamycin nephropathy rat model. BMC Nephrol. 2018;19:140.
Camara AKS, Zhou Y, Wen PC, Tajkhorshid E, Kwok WM. Mitochondrial VDAC1: a key gatekeeper as potential therapeutic target. Front Physiol. 2017;8:460.
D’Eletto M, Rossin F, Occhigrossi L, Farrace MG, Faccenda D, Desai R, et al. Transglutaminase type 2 regulates ER-mitochondria contact sites by interacting with GRP75. Cell Rep. 2018;25:3573–81.e4.
Panahi G, Pasalar P, Zare M, Rizzuto R, Meshkani R. MCU-knockdown attenuates high glucose-induced inflammation through regulating MAPKs/NF-κB pathways and ROS production in HepG2 cells. PLoS ONE. 2018;13:e0196580.
Zhang E, Mohammed Al-Amily I, Mohammed S, Luan C, Asplund O, Ahmed M, et al. Preserving insulin secretion in diabetes by inhibiting VDAC1 overexpression and surface translocation in β cells. Cell Metab. 2019;29:64–77.e66.
Espin JC, Larrosa M, Garcia-Conesa MT, Tomas-Barberan F. Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: the evidence so far. Evid Based Complement Altern Med. 2013;2013:270418.
Garcia-Villalba R, Beltran D, Espin JC, Selma MV, Tomas-Barberan FA. Time course production of urolithins from ellagic acid by human gut microbiota. J Agric Food Chem. 2013;61:8797–806.
Selma MV, Beltran D, Luna MC, Romo-Vaquero M, Garcia-Villalba R, Mira A, et al. Isolation of human intestinal bacteria capable of producing the bioactive metabolite isourolithin A from ellagic acid. Front Microbiol. 2017;8:1521.
Selma MV, Tomas-Barberan FA, Beltran D, Garcia-Villalba R, Espin JC. Gordonibacter urolithinfaciens sp. nov., a urolithin-producing bacterium isolated from the human gut. Int J Syst Evol Microbiol. 2014;64(Pt 7):2346–52.
Piwowarski JP, Granica S, Zwierzynska M, Stefanska J, Schopohl P, Melzig MF, et al. Role of human gut microbiota metabolism in the anti-inflammatory effect of traditionally used ellagitannin-rich plant materials. J Ethnopharmacol. 2014;155:801–9.
Bourjeily G, Danilack VA, Bublitz MH, Lipkind H, Muri J, Caldwell D, et al. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort. Sleep Med. 2017;38:50–57.
Yuan T, Ma H, Liu W, Niesen DB, Shah N, Crews R, et al. Pomegranate’s neuroprotective effects against Alzheimer’s disease are mediated by urolithins, its ellagitannin-gut microbial derived metabolites. ACS Chem Neurosci. 2016;7:26–33.
Kujawska M, Jourdes M, Kurpik M, Szulc M, Szaefer H, Chmielarz P, et al. Neuroprotective effects of pomegranate juice against Parkinson’s disease and presence of ellagitannins-derived metabolite-urolithin A-in the brain. Int J Mol Sci. 2019;21:202.
Verzelloni E, Pellacani C, Tagliazucchi D, Tagliaferri S, Calani L, Costa LG, et al. Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol Nutr Food Res. 2011;55(Suppl 1):S35–43.
Gonzalez-Sarrias A, Nunez-Sanchez MA, Tomas-Barberan FA, Espin JC. Neuroprotective effects of bioavailable polyphenol-derived metabolites against oxidative stress-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. J Agric Food Chem. 2017;65:752–8.
Lee G, Park JS, Lee EJ, Ahn JH, Kim HS. Anti-inflammatory and antioxidant mechanisms of urolithin B in activated microglia. Phytomedicine. 2019;55:50–7.
Ryu D, Mouchiroud L, Andreux PA, Katsyuba E, Moullan N, Nicolet-Dit-Felix AA, et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016;22:879–88.
Sun Y, Vashisht AA, Tchieu J, Wohlschlegel JA, Dreier L. Voltage-dependent anion channels (VDACs) recruit Parkin to defective mitochondria to promote mitochondrial autophagy. J Biol Chem. 2012;287:40652–60.
Cerda B, Periago P, Espin JC, Tomas-Barberan FA. Identification of urolithin a as a metabolite produced by human colon microflora from ellagic acid and related compounds. J Agric Food Chem. 2005;53:5571–6.
Zhao W, Shi F, Guo Z, Zhao J, Song X, Yang H. Metabolite of ellagitannins, urolithin A induces autophagy and inhibits metastasis in human sw620 colorectal cancer cells. Mol Carcinog. 2018;57:193–200.
Kasimsetty SG, Bialonska D, Reddy MK, Ma G, Khan SI, Ferreira D. Colon cancer chemopreventive activities of pomegranate ellagitannins and urolithins. J Agric Food Chem. 2010;58:2180–7.
Boakye YD, Groyer L, Heiss EH. An increased autophagic flux contributes to the anti-inflammatory potential of urolithin A in macrophages. Biochim Biophys Acta Gen Subj. 2018;1862:61–70.
Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharm. 2014;18:1–14.
Dassanayaka S, Readnower RD, Salabei JK, Long BW, Aird AL, Zheng YT, et al. High glucose induces mitochondrial dysfunction independently of protein O-GlcNAcylation. Biochem J. 2015;467:115–26.
Banerjee PS, Ma J, Hart GW. Diabetes-associated dysregulation of O-GlcNAcylation in rat cardiac mitochondria. Proc Natl Acad Sci USA. 2015;112:6050–5.
Detaille D, Guigas B, Chauvin C, Batandier C, Fontaine E, Wiernsperger N, et al. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes. 2005;54:2179–87.
Ji L, Liu F, Jing Z, Huang Q, Zhao Y, Cao H, et al. MICU1 alleviates diabetic cardiomyopathy through mitochondrial Ca2+-dependent antioxidant response. Diabetes. 2017;66:1586–1600.
Zhang S, Al-Maghout T, Cao H, Pelzl L, Salker MS, Veldhoen M, et al. Gut bacterial metabolite urolithin A (UA) mitigates Ca2+ entry in T cells by regulating miR-10a-5p. Front Immunol. 2019;10:1737.
Bayle M, Neasta J, Dall’Asta M, Gautheron G, Virsolvy A, Quignard JF, et al. The ellagitannin metabolite urolithin C is a glucose-dependent regulator of insulin secretion through activation of L-type calcium channels. Br J Pharm. 2019;176:4065–78.
Gong Z, Huang J, Xu B, Ou Z, Zhang L, Lin X, et al. Urolithin A attenuates memory impairment and neuroinflammation in APP/PS1 mice. J Neuroinflammation. 2019;16:62.
Selma MV, Gonzalez-Sarrias A, Salas-Salvado J, Andres-Lacueva C, Alasalvar C, Orem A, et al. The gut microbiota metabolism of pomegranate or walnut ellagitannins yields two urolithin-metabotypes that correlate with cardiometabolic risk biomarkers: comparison between normoweight, overweight-obesity and metabolic syndrome. Clin Nutr. 2018;37:897–905.
Toney AM, Fan R, Xian Y, Chaidez V, Ramer-Tait AE, Chung S, et al. A gut metabolite, improves insulin sensitivity through augmentation of mitochondrial function and biogenesis. Obes (Silver Spring). 2019;27:612–20.
Greenberg CS, Birckbichler PJ, Rice RH. Transglutaminases: multifunctional cross-linking enzymes that stabilize tissues. FASEB J. 1991;5:3071–7.
Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–56.
Piacentini M, D’Eletto M, Falasca L, Farrace MG, Rodolfo C. Transglutaminase 2 at the crossroads between cell death and survival. Adv Enzymol Relat Areas Mol Biol. 2011;78:197–246.
Facchiano F, Facchiano A, Facchiano AM. The role of transglutaminase-2 and its substrates in human diseases. Front Biosci. 2006;11:1758–73.
Ludvigsen TP, Olsen LH, Pedersen HD, Christoffersen BO, Jensen LJ. Hyperglycemia-induced transcriptional regulation of ROCK1 and TGM2 expression is involved in small artery remodeling in obese diabetic Gottingen Minipigs. Clin Sci (Lond). 2019;133:2499–516.
Schelling JR. Tissue transglutaminase inhibition as treatment for diabetic glomerular scarring: it’s good to be glueless. Kidney Int. 2009;76:363–5.
Dabir P, Marinic TE, Krukovets I, Stenina OI. Aryl hydrocarbon receptor is activated by glucose and regulates the thrombospondin-1 gene promoter in endothelial cells. Circ Res. 2008;102:1558–65.
Muku GE, Murray IA, Espin JC, Perdew GH. Urolithin A is a dietary microbiota-derived human aryl hydrocarbon receptor antagonist. Metabolites. 2018;8:86.
Andreola F, Calvisi DF, Elizondo G, Jakowlew SB, Mariano J, Gonzalez FJ, et al. Reversal of liver fibrosis in aryl hydrocarbon receptor null mice by dietary vitamin A depletion. Hepatology. 2004;39:157–66.
Singh R, Chandrashekharappa S, Bodduluri SR, Baby BV, Hegde B, Kotla NG, et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun. 2019;10:89.
Nukaya M, Lin BC, Glover E, Moran SM, Kennedy GD, Bradfield CA. The aryl hydrocarbon receptor-interacting protein (AIP) is required for dioxin-induced hepatotoxicity but not for the induction of the Cyp1a1 and Cyp1a2 genes. J Biol Chem. 2010;285:35599–605.
Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol. 2014;32:403–32.
Trivellin G, Korbonits M. AIP and its interacting partners. J Endocrinol. 2011;210:137–55.
Martino Adami PV, Nichtova Z, Weaver DB, Bartok A, Wisniewski T, Jones DR, et al. Perturbed mitochondria-ER contacts in live neurons that model the amyloid pathology of Alzheimer’s disease. J Cell Sci. 2019;132:jcs229906.
Area-Gomez E, de Groof AJ, Boldogh I, Bird TD, Gibson GE, Koehler CM, et al. Presenilins are enriched in endoplasmic reticulum membranes associated with mitochondria. Am J Pathol. 2009;175:1810–6.
Calvo-Rodriguez M, Hernando-Perez E, Nunez L, Villalobos C. Amyloid β oligomers increase ER-mitochondria Ca2+ cross talk in young hippocampal neurons and exacerbate aging-induced intracellular Ca2+ remodeling. Front Cell Neurosci. 2019;13:22.
Min B, Chung KC. New insight into transglutaminase 2 and link to neurodegenerative diseases. BMB Rep. 2018;51:5–13.
Norlund MA, Lee JM, Zainelli GM, Muma NA. Elevated transglutaminase-induced bonds in PHF tau in Alzheimer’s disease. Brain Res. 1999;851:154–63.
Fa M, Orozco IJ, Francis YI, Saeed F, Gong Y, Arancio O. Preparation of oligomeric β-amyloid 1-42 and induction of synaptic plasticity impairment on hippocampal slices. J Vis Exp. 2010;2010:e1884.
Acknowledgements
This research was supported by National R&D Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2020R1A2B5B02002442, NRF-2018R1D1A1B07050212) and BK21 PLUS Program for Creative Veterinary Science Research.
Author information
Authors and Affiliations
Contributions
HJL: Conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing. YHJ: Conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing. GEC, JSK, CWC, JRL, SYK, and JHY: Collection of data. SJL: Data analysis and interpretation, manuscript writing. HJH: Conception and design, data analysis and interpretation, and manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by: M. Piacentini
Rights and permissions
About this article
Cite this article
Lee, H.J., Jung, Y.H., Choi, G.E. et al. Urolithin A suppresses high glucose-induced neuronal amyloidogenesis by modulating TGM2-dependent ER-mitochondria contacts and calcium homeostasis. Cell Death Differ 28, 184–202 (2021). https://doi.org/10.1038/s41418-020-0593-1
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41418-020-0593-1
This article is cited by
-
Phosphatidate phosphatase Lipin1 alters mitochondria-associated endoplasmic reticulum membranes (MAMs) homeostasis: effects which contribute to the development of diabetic encephalopathy
Journal of Neuroinflammation (2025)
-
Mitochondrial Damage and Autophagy Dysregulation in Alzheimer’s Disease: Mechanisms and Therapeutic Opportunities
Neurochemical Research (2025)
-
Osteoblastic ferroptosis inhibition by small-molecule promoting GPX4 activation for peri-prosthetic osteolysis therapy
Journal of Nanobiotechnology (2024)
-
The potential role of gut microbiota-derived metabolites as regulators of metabolic syndrome-associated mitochondrial and endolysosomal dysfunction in Alzheimer’s disease
Experimental & Molecular Medicine (2024)
-
E3 ubiquitin ligase TRIM31 alleviates dopaminergic neurodegeneration by promoting proteasomal degradation of VDAC1 in Parkinson’s Disease model
Cell Death & Differentiation (2024)