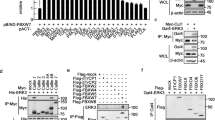
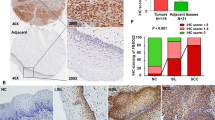
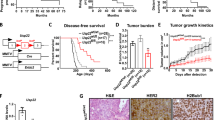
Abstract
SKP1-CUL1-F-box (SCF) ubiquitin ligases play fundamental roles in cellular functions. Typically, substrate phosphorylation is required for SCF recognition and subsequent degradation. However, phospho-dependent substrates remain largely unidentified. Here, using quantitative phoshoproteome approach, we performed a system-wide investigation of phospho-dependent SCF substrates. This strategy identified diverse phospho-dependent candidates. Biochemical verification revealed a mechanism by which SCFFBXO22 recognizes the motif XXPpSPXPXX as a conserved phosphodegron to target substrates for destruction. We further demonstrated BAG3, a HSP70 co-chaperone, is a bona fide substrate of SCFFBXO22. FBXO22 mediates BAG3 ubiquitination and degradation that requires ERK-dependent BAG3 phosphorylation at S377. FBXO22 depletion or expression of a stable BAG3 S377A mutant promotes tumor growth via defects in apoptosis and cell cycle progression in vitro and in vivo. In conclusion, our study identified broad phosphorylation-dependent SCF substrates and demonstrated a phosphodegron recognized by FBXO22 and a novel ERK-FBXO22-BAG3 axis involved in tumorigenesis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the supplementary materials. Mass spectrometry data have been deposited to the iProX (84), a full member of the ProteomeXchange consortium (http://www.iprox.cn), accession ID: IPX0001809000.
References
Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403.
Jang HH. Regulation of protein degradation by proteasomes in cancer. J Cancer Prev. 2018;23:153–61.
Varshavsky A. Naming a targeting signal. Cell. 1991;64:13–5.
Lucas X, Ciulli A. Recognition of substrate degrons by E3 ubiquitin ligases and modulation by small-molecule mimicry strategies. Curr Opin Struct Biol. 2017;44:101–10.
Sarikas A, Hartmann T, Pan ZQ. The cullin protein family. Genome Biol. 2011;12:220.
Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:739–51.
Feldman RMR, Correll CC, Kaplan KB, Deshaies RJ. A complex of Cdc4p, Skp1p, and Cdc53p/cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell. 1997;91:221–30.
Reitsma JM, Liu X, Reichermeier KM, Moradian A, Sweredoski MJ, Hess S, et al. Composition and regulation of the cellular repertoire of SCF ubiquitin ligases. Cell. 2017;171:1326–39.e1314.
Skowyra D, Craig KL, Tyers M, Elledge SJ, Harper JW. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell. 1997;91:209–19.
Ang XL, Wade Harper J. SCF-mediated protein degradation and cell cycle control. Oncogene. 2005;24:2860–70.
Jin J, Shirogane T, Xu L, Nalepa G, Qin J, Elledge SJ, et al. SCFbeta-TRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes Dev. 2003;17:3062–74.
Ye X, Nalepa G, Welcker M, Kessler BM, Spooner E, Qin J, et al. Recognition of phosphodegron motifs in human cyclin E by the SCF(Fbw7) ubiquitin ligase. J Biol Chem. 2004;279:50110–9.
Orlicky S, Tang X, Willems A, Tyers M, Sicheri F. Structural basis for phosphodependent substrate selection and orientation by the SCFCdc4 ubiquitin ligase. Cell. 2003;112:243–56.
Emanuele MJ, Elia AE, Xu Q, Thoma CR, Izhar L, Leng Y, et al. Global identification of modular cullin-RING ligase substrates. Cell. 2011;147:459–74.
Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell. 2011;44:325–40.
Liu B, Jiang S, Li M, Xiong X, Zhu M, Li D, et al. Proteome-wide analysis of USP14 substrates revealed its role in hepatosteatosis via stabilization of FASN. Nat Commun. 2018;9:4770.
Tan MK, Lim HJ, Bennett EJ, Shi Y, Harper JW. Parallel SCF adaptor capture proteomics reveals a role for SCFFBXL17 in NRF2 activation via BACH1 repressor turnover. Mol Cell. 2013;52:9–24.
Kim TY, Siesser PF, Rossman KL, Goldfarb D, Mackinnon K, Yan F, et al. Substrate trapping proteomics reveals targets of the betaTrCP2/FBXW11 ubiquitin ligase. Mol Cell Biol. 2015;35:167–81.
Takayama S, Reed JC. Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol. 2001;3:E237–241.
Behl C, Breaking BAG. The co-chaperone BAG3 in health and disease. Trends Pharm Sci. 2016;37:672–88.
De Marco M, Basile A, Iorio V, Festa M, Falco A, Ranieri B, et al. Role of BAG3 in cancer progression: a therapeutic opportunity. Semin Cell Dev Biol. 2018;78:85–92.
Meister-Broekema M, Freilich R, Jagadeesan C, Rauch JN, Bengoechea R, Motley WW, et al. Myopathy associated BAG3 mutations lead to protein aggregation by stalling Hsp70 networks. Nat Commun. 2018;9:5342.
Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–6.
Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369–81.
Scott DC, Rhee DY, Duda DM, Kelsall IR, Olszewski JL, Paulo JA, et al. Two distinct types of E3 ligases work in unison to regulate substrate ubiquitylation. Cell. 2016;166:1198–214.e1124.
Yalla K, Elliott C, Day JP, Findlay J, Barratt S, Hughes ZA, et al. FBXW7 regulates DISC1 stability via the ubiquitin-proteosome system. Mol Psychiatry. 2018;23:1278–86.
Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal. 2010;3:ra3.
Biswas M, Phan D, Watanabe M, Chan JY. The Fbw7 tumor suppressor regulates nuclear factor E2-related factor 1 transcription factor turnover through proteasome-mediated proteolysis. J Biol Chem. 2011;286:39282–9.
Hishiya A, Kitazawa T, Takayama S. BAG3 and Hsc70 interact with actin capping protein CapZ to maintain myofibrillar integrity under mechanical stress. Circ Res. 2010;107:1220–31.
Sun R, Xie HY, Qian JX, Huang YN, Yang F, Zhang FL, et al. FBXO22 possesses both protumorigenic and antimetastatic roles in breast cancer progression. Cancer Res. 2018;78:5274–86.
Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–38.
Xue L, Wang P, Cao P, Zhu JK, Tao WA. Identification of extracellular signal-regulated kinase 1 (ERK1) direct substrates using stable isotope labeled kinase assay-linked phosphoproteomics. Mol Cell Proteom. 2014;13:3199–210.
Broutier L, Mastrogiovanni G, Verstegen MM, Francies HE, Gavarro LM, Bradshaw CR, et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23:1424–35.
Grant CE, Bailey TL, Noble WS. FIMO: scanning for occurrences of a given motif. Bioinformatics. 2011;27:1017–8.
Low TY, Peng M, Magliozzi R, Mohammed S, Guardavaccaro D, Heck AJ. A systems-wide screen identifies substrates of the SCFbetaTrCP ubiquitin ligase. Sci Signal. 2014;7:rs8.
Fremin C, Guegan JP, Plutoni C, Mahaffey J, Philips MR, Emery G, et al. ERK1/2-induced phosphorylation of R-Ras GTPases stimulates their oncogenic potential. Oncogene. 2016;35:5692–8.
Huang X, Yan J, Zhang M, Wang YF, Chen Y, Fu XH, et al. Targeting epigenetic crosstalk as a therapeutic strategy for EZH2-Aberrant solid tumors. Cell. 2018;175:186−+.
Kuchay S, Duan S, Schenkein E, Peschiaroli A, Saraf A, Florens L, et al. FBXL2- and PTPL1-mediated degradation of p110-free p85beta regulatory subunit controls the PI(3)K signalling cascade. Nat Cell Biol. 2013;15:472–80.
Jeong YT, Simoneschi D, Keegan S, Melville D, Adler NS, Saraf A, et al. The ULK1-FBXW5-SEC23B nexus controls autophagy. Elife. 2018;7:e42253.
Lignitto L, LeBoeuf SE, Homer H, Jiang S, Askenazi M, Karakousi TR, et al. Nrf2 activation promotes lung cancer metastasis by inhibiting the degradation of bach1. Cell. 2019;178:316–29.e318.
Bai J, Wu K, Cao MH, Yang Y, Pan Y, Liu H, et al. SCF(FBXO22) targets HDM2 for degradation and modulates breast cancer cell invasion and metastasis. Proc Natl Acad Sci USA. 2019;116:11754–63.
Johmura Y, Maeda I, Suzuki N, Wu W, Goda A, Morita M, et al. Fbxo22-mediated KDM4B degradation determines selective estrogen receptor modulator activity in breast cancer. J Clin Invest. 2018;128:5603–19.
Thingholm TE, Jorgensen TJ, Jensen ON, Larsen MR. Highly selective enrichment of phosphorylated peptides using titanium dioxide. Nat Protoc. 2006;1:1929–35.
Nie L, Shuai L, Zhu M, Liu P, Xie ZF, Jiang S, et al. The landscape of histone modifications in a high-fat diet-induced obese (DIO) mouse model. Mol Cell Proteom. 2017;16:1324–34.
van Zundert GCP, Rodrigues J, Trellet M, Schmitz C, Kastritis PL, Karaca E, et al. The HADDOCK2.2 Web Server: user-friendly integrative modeling of biomolecular complexes. J Mol Biol. 2016;428:720–5.
Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–72.
Wisniewski JR, Mann M. A proteomics approach to the protein normalization problem: selection of unvarying proteins for MS-based proteomics and western blotting. J Proteome Res. 2016;15:2321–6.
Larance M, Ahmad Y, Kirkwood KJ, Ly T, Lamond AI. Global subcellular characterization of protein degradation using quantitative proteomics. Mol Cell Proteom. 2013;12:638–50.
Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57.
Lachmann A, Ma’ayan A. KEA: kinase enrichment analysis. Bioinformatics. 2009;25:684–6.
Cheng A, Grant CE, Noble WS, Bailey TL. MoMo: discovery of statistically significant post-translational modification motifs. Bioinformatics. 2019;35:2774–82.
Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–90.
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Chatr-Aryamontri A, Oughtred R, Boucher L, Rust J, Chang C, Kolas NK, et al. The BioGRID interaction database: 2017 update. Nucleic Acids Res. 2017;45:D369–D379.
Hornbeck PV, Kornhauser JM, Tkachev S, Zhang B, Skrzypek E, Murray B, et al. PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res. 2012;40(Database issue):D261–70.
Tate JG, Bamford S, Jubb HC, Sondka Z, Beare DM, Bindal N, et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2019;47:D941–7.
Hjerpe R, Aillet F, Lopitz-Otsoa F, Lang V, England P, Rodriguez MS. Efficient protection and isolation of ubiquitylated proteins using tandem ubiquitin-binding entities. EMBO Rep. 2009;10:1250–8.
Author information
Authors and Affiliations
Contributions
Conceptualization, MT and BL; Methodology, PL, XC, SL, and XJ; Formal Analysis, XC, LZ, DN, and ZL; Investigation, PL, XC, SL, XJ, WD, LZ, XW, YC, JJ, WL, and LP; Resources, MT, BL, MH, and JZ; Data Curation, PL, XC, SL, and XJ; Writing, MT, BL, PL, and XC; Visualization, PL, XC, SL, WD, and XJ; Supervision, MT and BL; Funding Acquisition, MT, BL, WD, and JZ; All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics statement
Our studies did not include human participants, human data, or human tissue. The animal studies were conducted in compliance with the Institutional Animal Care and Use Committee of institutional ethical guidelines in Shanghai Institute of Materia Medica, Chinese Academy of Sciences (2019-03-GMY-10).
Funding statement
This work was supported by the Natural Science Foundation of China (Nos. 32071432, 81872888, 81821005, 91753203, 81773018, 71473074), National Key R&D Program of China (No. 2020YFE020220), the National Science & Technology Major Project “Key New Drug Creation and Manufacturing Program” (No. 2018ZX09711002-004), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA12050406), the Shanghai Science and Technology Committee (No. 19JC1416300), Innovative Research Team of High-Level Local Universities in Shanghai (SSMU-ZDCX20181202) and the K. C. Wong Education Foundation.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by A. Degterev
Rights and permissions
About this article
Cite this article
Liu, P., Cong, X., Liao, S. et al. Global identification of phospho-dependent SCF substrates reveals a FBXO22 phosphodegron and an ERK-FBXO22-BAG3 axis in tumorigenesis. Cell Death Differ 29, 1–13 (2022). https://doi.org/10.1038/s41418-021-00827-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-021-00827-7
This article is cited by
-
Phosphorylation-dependent STAMBP drives the progression of pancreatic ductal adenocarcinoma by deubiquitinating and stabilizing BAG3
Cell Death & Differentiation (2026)
-
F-box proteins in cancer: from cancer cells to the tumor microenvironment
Cell Communication and Signaling (2025)
-
METTL21A promotes hepatocellular carcinoma progression via methylating and stabilizing BAG3
npj Precision Oncology (2025)
-
BAG3 promotes proliferation and migration of arterial smooth muscle cells by regulating STAT3 phosphorylation in diabetic vascular remodeling
Cardiovascular Diabetology (2024)
-
Promotion of stem cell-like phenotype of lung adenocarcinoma by FAM83A via stabilization of ErbB2
Cell Death & Disease (2024)