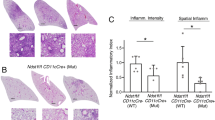
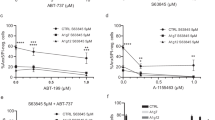
Abstract
Inflammation is a natural defence mechanism of the body to protect against pathogens. It is induced by immune cells, such as macrophages and neutrophils, which are rapidly recruited to the site of infection, mediating host defence. The processes for eliminating inflammatory cells after pathogen clearance are critical in preventing sustained inflammation, which can instigate diverse pathologies. During chronic inflammation, the excessive and uncontrollable activity of the immune system can cause extensive tissue damage. New therapies aimed at preventing this over-activity of the immune system could have major clinical benefits. Here, we investigated the role of the pro-survival Bcl-2 family member A1 in the survival of inflammatory cells under normal and inflammatory conditions using murine models of lung and peritoneal inflammation. Despite the robust upregulation of A1 protein levels in wild-type cells upon induction of inflammation, the survival of inflammatory cells was not impacted in A1-deficient mice compared to wild-type controls. These findings indicate that A1 does not play a major role in immune cell homoeostasis during inflammation and therefore does not constitute an attractive therapeutic target for such morbidities.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20.
Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–406.
Vogler M. BCL2A1: the underdog in the BCL2 family. Cell Death Differ. 2012;19:67–74.
Lin EY, Orlofsky A, Berger MS, Prystowsky MB. Characterization of A1, a novel hemopoietic-specific early-response gene with sequence similarity to Bcl-2. J Immunol. 1993;151:1979–88.
Karsan A, Yee E, Kaushansky K, Harlan JM. Cloning of human Bcl-2 homologue: inflammatory cytokines induce human A1 in cultured endothelial cells. Blood. 1996;87:3089–96.
Zong WX, Edelstein LC, Chen C, Bash J, Gelinas C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 1999;13:382–7.
Hatakeyama S, Hamasaki A, Negishi I, Loh DY, Sendo F, Nakayama K, et al. Multiple gene duplication and expression of mouse bcl-2-related genes, A1. Int Immunol. 1998;10:631–7.
Hamasaki A, Sendo F, Nakayama K, Ishida N, Negishi I, Nakayama K, et al. Accelerated neutrophil apoptosis in mice lacking A1-a, a subtype of the bcl-2-related A1 gene. J Exp Med. 1998;188:1985–92.
Xiang Z, Ahmed AA, Moller C, Nakayama K, Hatakeyama S, Nilsson G. Essential role of the prosurvival bcl-2 homologue A1 in mast cell survival after allergic activation. J Exp Med. 2001;194:1561–9.
Schenk RL, Tuzlak S, Carrington EM, Zhan Y, Heinzel S, Teh CE, et al. Characterisation of mice lacking all functional isoforms of the pro-survival BCL-2 family member A1 reveals minor defects in the haematopoietic compartment. Cell Death Differ. 2017;24:534–45.
Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–24.
O’Donnell JA, Kennedy CL, Pellegrini M, Nowell CJ, Zhang JG, O’Reilly LA, et al. Fas regulates neutrophil lifespan during viral and bacterial infection. J Leukoc Biol. 2015;97:321–6.
Luo HR, Loison F. Constitutive neutrophil apoptosis: mechanisms and regulation. Am J Hematol. 2008;83:288–95.
Koedel U, Frankenberg T, Kirschnek S, Obermaier B, Hacker H, Paul R, et al. Apoptosis is essential for neutrophil functional shutdown and determines tissue damage in experimental pneumococcal meningitis. PLoS Pathogens 2009;5:e1000461.
Dzhagalov I, St John A, He YW. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109:1620–6.
Akgul C, Moulding DA, Edwards SW. Molecular control of neutrophil apoptosis. FEBS Lett. 2001;487:318–22.
Herold MJ, Zeitz J, Pelzer C, Kraus C, Peters A, Wohlleben G, et al. The stability and anti-apoptotic function of A1 are controlled by its C terminus. J Biol Chem. 2006;281:13663–71.
Adams KW, Cooper GM. Rapid turnover of MCL-1 couples translation to cell survival and apoptosis. J Biol Chem. 2007;282:6192–6200.
Lang MJ, Brennan MS, O’Reilly LA, Ottina E, Czabotar PE, Whitlock E, et al. Characterisation of a novel A1-specific monoclonal antibody. Cell Death Dis. 2014;5:e1553.
Vier J, Groth M, Sochalska M, Kirschnek S. The anti-apoptotic Bcl-2 family protein A1/Bfl-1 regulates neutrophil survival and homeostasis and is controlled via PI3K and JAK/STAT signaling. Cell Death Dis. 2016;7:e2103.
Schenk RL, Gangoda L, Lawlor KE, O’Reilly LA, Strasser A, Herold MJ. The pro-survival Bcl-2 family member A1 delays spontaneous and FAS ligand-induced apoptosis of activated neutrophils. Cell Death Dis. 2020;11:474.
Best SA, De Souza DP, Kersbergen A, Policheni AN, Dayalan S, Tull D, et al. Synergy between the KEAP1/NRF2 and PI3K pathways drives non-small-cell lung cancer with an altered immune microenvironment. Cell Metab. 2018;27:935–943 e934.
Kukavica-Ibrulj I, Levesque RC. Animal models of chronic lung infection with Pseudomonas aeruginosa: useful tools for cystic fibrosis studies. Lab Anim. 2008;42:389–412.
Bayes HK, Ritchie N, Irvine S, Evans TJ. A murine model of early Pseudomonas aeruginosa lung disease with transition to chronic infection. Sci Rep. 2016;6:35838.
Kathania M, Raje CI, Raje M, Dutta RK, Majumdar S. Bfl-1/A1 acts as a negative regulator of autophagy in mycobacteria infected macrophages. Int J Biochem Cell Biol. 2011;43:573–85.
Kausalya S, Somogyi R, Orlofsky A, Prystowsky MB. Requirement of A1-a for bacillus Calmette-Guerin-mediated protection of macrophages against nitric oxide-induced apoptosis. J Immunol. 2001;166:4721–7.
Orlofsky A, Somogyi RD, Weiss LM, Prystowsky MB. The murine antiapoptotic protein A1 is induced in inflammatory macrophages and constitutively expressed in neutrophils. J Immunol. 1999;163:412–9.
Doerflinger M, Glab J, Puthalakath H. Experimental in vivo sepsis models to monitor immune cell apoptosis and survival in laboratory mice. Methods Mol Biol. 2016;1419:69–81.
Russell JH, Rush B, Weaver C, Wang R. Mature T cells of autoimmune LPR/LPR mice have a defect in antigen-stimulated suicide. Proc Natl Acad Sci USA. 1993;90:4409–13.
Lee HH, Dadgostar H, Cheng Q, Shu J, Cheng G. NF-kappaB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc Natl Acad Sci USA. 1999;96:9136–41.
Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 2000;14:23–27.
Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Nakayama K, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–10.
Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–40.
Kotschy A, Szlavik Z, Murray J, Davidson J, Maragno AL, Le Toumelin-Braizat G, et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538:477–82.
Merino D, Kelly GL, Lessene G, Wei AH, Roberts AW, Strasser A. BH3-mimetic drugs: blazing the trail for new cancer medicines. Cancer Cell. 2018;34:879–91.
Cox G, Crossley J, Xing Z. Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am J Respir Cell Mol Biol. 1995;12:232–7.
Cakarova L, Marsh LM, Wilhelm J, Mayer K, Grimminger F, Seeger W, et al. Macrophage tumor necrosis factor-alpha induces epithelial expression of granulocyte-macrophage colony-stimulating factor: impact on alveolar epithelial repair. Am J Respir Crit Care Med. 2009;180:521–32.
Verschelde C, Walzer T, Galia P, Biemont MC, Quemeneur L, Revillard JP, et al. A1/Bfl-1 expression is restricted to TCR engagement in T lymphocytes. Cell Death Differ. 2003;10:1059–67.
Tuzlak S, Schenk RL, Vasanthakumar A, Preston SP, Haschka MD, Zotos D, et al. The BCL-2 pro-survival protein A1 is dispensable for T cell homeostasis on viral infection. Cell Death Differ. 2017;24:523–33.
Ottina E, Tischner D, Herold MJ, Villunger A. A1/Bfl-1 in leukocyte development and cell death. Exp Cell Res. 2012;318:1291–303.
Doeing DC, Borowicz JL, Crockett ET. Gender dimorphism in differential peripheral blood leukocyte counts in mice using cardiac, tail, foot, and saphenous vein puncture methods. BMC Clin Pathol. 2003;3:3.
Mestas J, Hughes CCW. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–8.
Wiener-Kronish JP, Albertine KH, Matthay MA. Differential responses of the endothelial and epithelial barriers of the lung in sheep to Escherichia coli endotoxin. J Clin Invest. 1991;88:864–75.
Bragonzi A. Murine models of acute and chronic lung infection with cystic fibrosis pathogens. Int J Med Microbiol. 2010;300:584–93.
Murando F, Peloso A, Cobianchi L. Experimental abdominal sepsis: sticking to an awkward but still useful translational model. Mediators Inflamm. 2019;2019:8971036.
Gonnert FA, Recknagel P, Seidel M, Jbeily N, Dahlke K, Bockmeyer CL, et al. Characteristics of clinical sepsis reflected in a reliable and reproducible rodent sepsis model. J Surg Res. 2011;170:e123–134.
Lee MJ, Kim K, Jo YH, Lee JH, Hwang JE. Dose-dependent mortality and organ injury in a cecal slurry peritonitis model. J Surg Res. 2016;206:427–34.
Best SA, Kersbergen A, Asselin-Labat ML, Sutherland KD. Combining cell type-restricted adenoviral targeting with immunostaining and flow cytometry to identify cells-of-origin of lung cancer. Methods Mol Biol. 2018;1725:15–29.
Acknowledgements
We acknowledge the invaluable contributions of the animal caretaker staff for animal husbandry and the flow cytometry facility of WEHI.
Funding
This work was supported by grants and fellowships from the Australian National Health and Medical Research Council (NHMRC) (Project Grants 1186575, 1159658 and 1145728 to MJH, 1143105 to MJH and AS), Programme Grant 1016701 to AS and Fellowships 1020363 to AS, 1156095 to MJH, the Leukaemia and Lymphoma Society of America (LLS SCOR 7015-18 to AS and MJH), the Cancer Council of Victoria (project grant 1147328 to MJH, 1052309 to AS and Venture Grant to MJH and AS), the Australian Phenomics Network (to MJH), the Leukaemia and Lymphoma Society Grant #7001-13 to AS; the estate of Anthony (Toni) Redstone OAM and The Craig Perkins Cancer Research Foundation; and operational infrastructure grants through the Australian Government NHMRCS IRIISS and the Victorian State Government Operational Infrastructure Support to AS and the CASS Foundation Science and Medicine Grant #9393 to LG. KF is the recipient of the Alex Gadomski Fellowship, funded by Maddie Riewoldt’s Vision. KDS is supported by the Peter and Julie Alston Centenary Fellowship.
Author information
Authors and Affiliations
Contributions
LG performed and designed most experiments and wrote the manuscript; RLS, SAB, CN, CL, DD, and KF helped to perform experiments and write the manuscript; AJ, HP, and KDS helped with discussions and advice on neutrophil experiments and write the manuscript; AS and MJH planned the project, were involved in experimental design and helped write the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
All animal experiments were approved by the Walter and Eliza Hall Institute of Medical Research (WEHI) and the La Trobe University (LTU) Animal Ethics Committees.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by G. Melino.
Supplementary information
Rights and permissions
About this article
Cite this article
Gangoda, L., Schenk, R.L., Best, S.A. et al. Absence of pro-survival A1 has no impact on inflammatory cell survival in vivo during acute lung inflammation and peritonitis. Cell Death Differ 29, 96–104 (2022). https://doi.org/10.1038/s41418-021-00839-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-021-00839-3
This article is cited by
-
Emerging biomarkers and potential therapeutics of the BCL-2 protein family: the apoptotic and anti-apoptotic context
Egyptian Journal of Medical Human Genetics (2024)
-
Contribution of A1 to macrophage survival in cooperation with MCL-1 and BCL-XL in a murine cell model of myeloid differentiation
Cell Death & Disease (2024)
-
Macrophage polarization and metabolism in atherosclerosis
Cell Death & Disease (2023)
-
Apoptotic cell death in disease—Current understanding of the NCCD 2023
Cell Death & Differentiation (2023)
-
What can we learn from mice lacking pro-survival BCL-2 proteins to advance BH3 mimetic drugs for cancer therapy?
Cell Death & Differentiation (2022)