Abstract
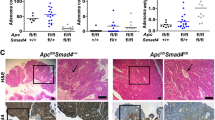
Cholangiocarcinoma (CCA), consisting of three subtypes-intrahepatic (iCCA), perihilar (pCCA), and distal (dCCA), is a highly aggressive cancer arising from the bile duct and has an extremely poor prognosis. Pemigatinib is the only FDA-approved targeted drug for CCA, and the CCA treatment options are substantially insufficient considering its poor prognosis and increasing morbidity. Here, we performed next-generation sequencing (NGS) of 15 pCCAs and 16 dCCAs and detected the expression of SMAD4, a frequently mutated gene, in 261 CCAs. By univariate and multivariate analyses, we identified Smad4 as a favorable prognostic biomarker in iCCA and pCCA. With in vitro and in vivo experiments, we demonstrated that Smad4 suppressed CCA proliferation, migration and invasion by inhibiting β-catenin-S675 phosphorylation and intranuclear translocation. We applied LC–MS/MS and multiple biochemical techniques and identified PP1A as the phosphatase in Smad4-mediated dephosphorylation of PAK1-T423, which is responsible for β-catenin-S675 phosphorylation. Moreover, we demonstrated that MYO18A is the PP1-interacting protein of PP1A for substrate recognition in CCA. MYO18A interacts with PP1A via its RVFFR motif and interacts with Smad4 via CC domain. Patients with coexpression of MYO18A and Smad4 have a more favorable prognosis than other patients. Smad4 enhances Pemigatinib efficiency, and Smad4 knockdown results in Pemigatinib resistance. In conclusion, coexpression of Smad4 and MYO18A is a favorable prognostic indicator for iCCA and pCCA. The Smad4-MYO18A-PP1A complex dephosphorylates PAK1-T423 and thus inhibits β-catenin-S675 phosphorylation and its intranuclear localization. Smad4 suppresses CCA proliferation, migration, invasion, and sensitivity to Pemigatinib by governing the phosphorylation and intracellular localization of β-catenin.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
No data was uploaded to the public database. All the data were available upon rational request.
References
Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma—evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95–111.
Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61:1657–69.
Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–88.
Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397:428–44.
Chen T, Li K, Liu Z, Liu J, Wang Y, Sun R, et al. WDR5 facilitates EMT and metastasis of CCA by increasing HIF-1alpha accumulation in Myc-dependent and independent pathways. Mol Ther. 2021;29:2134–50.
DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–62.
Li Z, Liu J, Chen T, Sun R, Liu Z, Qiu B, et al. HMGA1-TRIP13 axis promotes stemness and epithelial mesenchymal transition of perihilar cholangiocarcinoma in a positive feedback loop dependent on c-Myc. J Exp Clin Cancer Res. 2021;40:86.
Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–84.
David CJ, Huang YH, Chen M, Su J, Zou Y, Bardeesy N, et al. TGF-beta tumor suppression through a lethal EMT. Cell. 2016;164:1015–30.
Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85.
Inamoto S, Itatani Y, Yamamoto T, Minamiguchi S, Hirai H, Iwamoto M, et al. Loss of SMAD4 promotes colorectal cancer progression by accumulation of myeloid-derived suppressor cells through the CCL15-CCR1 chemokine axis. Clin Cancer Res. 2016;22:492–501.
Wang LH, Kim SH, Lee JH, Choi YL, Kim YC, Park TS, et al. Inactivation of SMAD4 tumor suppressor gene during gastric carcinoma progression. Clin Cancer Res. 2007;13:102–10.
Zhao M, Mishra L, Deng CX. The role of TGF-beta/SMAD4 signaling in cancer. Int J Biol Sci. 2018;14:111–23.
Wang F, Xia X, Yang C, Shen J, Mai J, Kim HC, et al. SMAD4 gene mutation renders pancreatic cancer resistance to radiotherapy through promotion of autophagy. Clin Cancer Res. 2018;24:3176–85.
Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003–10.
Wardell CP, Fujita M, Yamada T, Simbolo M, Fassan M, Karlic R, et al. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J Hepatol. 2018;68:959–69.
Freeman TJ, Smith JJ, Chen X, Washington MK, Roland JT, Means AL, et al. Smad4-mediated signaling inhibits intestinal neoplasia by inhibiting expression of beta-catenin. Gastroenterology. 2012;142:562–71.e2.
Voorneveld PW, Kodach LL, Jacobs RJ, van Noesel CJ, Peppelenbosch MP, Korkmaz KS, et al. The BMP pathway either enhances or inhibits the Wnt pathway depending on the SMAD4 and p53 status in CRC. Br J Cancer. 2015;112:122–30.
Zhi X, Lin L, Yang S, Bhuvaneshwar K, Wang H, Gusev Y, et al. betaII-Spectrin (SPTBN1) suppresses progression of hepatocellular carcinoma and Wnt signaling by regulation of Wnt inhibitor kallistatin. Hepatology. 2015;61:598–612.
Petit FG, Deng C, Jamin SP. Partial Mullerian Duct Retention in Smad4 Conditional Mutant Male Mice. Int J Biol Sci. 2016;12:667–76.
Moz S, Basso D, Bozzato D, Galozzi P, Navaglia F, Negm OH, et al. SMAD4 loss enables EGF, TGFbeta1 and S100A8/A9 induced activation of critical pathways to invasion in human pancreatic adenocarcinoma cells. Oncotarget. 2016;7:69927–44.
Liu Z, Sun R, Zhang X, Qiu B, Chen T, Li Z, et al. Transcription factor 7 promotes the progression of perihilar cholangiocarcinoma by inducing the transcription of c-Myc and FOS-like antigen 1. EBioMedicine. 2019;45:181–91.
Xu YF, Yang XQ, Lu XF, Guo S, Liu Y, Iqbal M, et al. Fibroblast growth factor receptor 4 promotes progression and correlates to poor prognosis in cholangiocarcinoma. Biochem Biophys Res Commun. 2014;446:54–60.
Sun R, Liu Z, Qiu B, Chen T, Li Z, Zhang X, et al. Annexin10 promotes extrahepatic cholangiocarcinoma metastasis by facilitating EMT via PLA2G4A/PGE2/STAT3 pathway. EBioMedicine. 2019;47:142–55.
Qiu B, Chen T, Sun R, Liu Z, Zhang X, Li Z, et al. Sprouty4 correlates with favorable prognosis in perihilar cholangiocarcinoma by blocking the FGFR-ERK signaling pathway and arresting the cell cycle. EBioMedicine. 2019;50:166–77.
Churi CR, Shroff R, Wang Y, Rashid A, Kang HC, Weatherly J, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PloS ONE. 2014;9:e115383.
Ong CK, Subimerb C, Pairojkul C, Wongkham S, Cutcutache I, Yu W, et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat Genet. 2012;44:690–3.
Montal R, Sia D, Montironi C, Leow WQ, Esteban-Fabro R, Pinyol R, et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J Hepatol. 2020;73:315–27.
Sia D, Hoshida Y, Villanueva A, Roayaie S, Ferrer J, Tabak B, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013;144:829–40.
Spirli C, Locatelli L, Morell CM, Fiorotto R, Morton SD, Cadamuro M, et al. Protein kinase A-dependent pSer(675) -beta-catenin, a novel signaling defect in a mouse model of congenital hepatic fibrosis. Hepatology. 2013;58:1713–23.
Barrios-Rodiles M, Brown KR, Ozdamar B, Bose R, Liu Z, Donovan RS, et al. High-throughput mapping of a dynamic signaling network in mammalian cells. Science. 2005;307:1621–5.
Bollen M, Peti W, Ragusa MJ, Beullens M. The extended PP1 toolkit: designed to create specificity. Trends Biochem Sci. 2010;35:450–8.
Paek H, Hwang JY, Zukin RS, Hebert JM. beta-Catenin-dependent FGF signaling sustains cell survival in the anterior embryonic head by countering Smad4. Dev Cell. 2011;20:689–99.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4.
Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–48.
Ferreira M, Beullens M, Bollen M, Van, Eynde A. Functions and therapeutic potential of protein phosphatase 1: insights from mouse genetics. Biochimica Biophys Acta Mol Cell Res. 2019;1866:16–30.
Stamos JL, Weis WI. The beta-catenin destruction complex. Cold Spring Harb Perspect Biol. 2013;5:a007898.
Li J, Huang X, Xu X, Mayo J, Bringas P Jr., Jiang R, et al. SMAD4-mediated WNT signaling controls the fate of cranial neural crest cells during tooth morphogenesis. Development. 2011;138:1977–89.
Jia L, Lee HS, Wu CF, Kundu J, Park SG, Kim RN, et al. SMAD4 suppresses AURKA-induced metastatic phenotypes via degradation of AURKA in a TGFbeta-independent manner. Mol Cancer Res. 2014;12:1779–95.
Salazar VS, Zarkadis N, Huang L, Watkins M, Kading J, Bonar S, et al. Postnatal ablation of osteoblast Smad4 enhances proliferative responses to canonical Wnt signaling through interactions with beta-catenin. J Cell Sci. 2013;126:5598–609.
Boulter L, Guest RV, Kendall TJ, Wilson DH, Wojtacha D, Robson AJ, et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Investig. 2015;125:1269–85.
Semenova G, Chernoff J. Targeting PAK1. Biochemical Soc Trans. 2017;45:79–88.
Demagny H, Araki T, De Robertis EM. The tumor suppressor Smad4/DPC4 is regulated by phosphorylations that integrate FGF, Wnt, and TGF-beta signaling. Cell Rep. 2014;9:688–700.
Funding
Our study was supported by Shandong University Multidisciplinary Research and Innovation Team of Young Scholars (Grant No. 2020QNQT002), National Natural Science Foundation of China (Grant No. 82072676, 82172791), China Postdoctoral Science Foundation (Grant No. 2020M682190, 2020M682195), Clinical Research Foundation of Shandong University (Grant No. 2020SDUCRCA018), Natural Science Foundation of Shandong Province (ZR2019MH008), Jinan City Science and Technology Development Program (Grant No. 201805017, 201805013), Clinical Research Innovation Fund Project (CXPJJH11800001–2018240), Hengrui Hepatobiliary and Pancreatic Foundation (Grant No.Y-2017-144), Key Research and Development Program of Shandong Province (Grant No. 2019GSF108254), Beijing Medical Award Foundation (YXJL-2020-0785-0967, YXJL-2020-0785-0968).
Author information
Authors and Affiliations
Contributions
JL, GR, KL, ZL, YW, TC, WM, XL, AS, and WZ performed experiments. XY reviewed pathology. BX, JC, SG, CP, and TZ collected the specimens and followed up the patients. ZZ collected the specimens and supervised the study. YX designed the study and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All the patients provided their consents for specimen obtainment and data analysis. The study protocol were approved and supervised by the Ethics Committee of Qilu Hospital of Shandong University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by P. Salomoni
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, J., Ren, G., Li, K. et al. The Smad4-MYO18A-PP1A complex regulates β-catenin phosphorylation and pemigatinib resistance by inhibiting PAK1 in cholangiocarcinoma. Cell Death Differ 29, 818–831 (2022). https://doi.org/10.1038/s41418-021-00897-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-021-00897-7
This article is cited by
-
The diagnostic and therapeutic potential of miR-3149 in osteoporotic fractures through SMAD4
Journal of Orthopaedic Surgery and Research (2025)
-
Targeting the ROCK2/UBA52/DRP1 axis enhances ferroptosis and overcomes pemigatinib resistance in Cholangiocarcinoma
Cell Death & Disease (2025)
-
Anagrelide and idarubicin combination induces GSDME-mediated pyroptosis as a potential therapy for high-PDE3A acute myeloid leukemia
Leukemia (2025)
-
AMDHD1 acts as a tumor suppressor and contributes to activation of TGF-β signaling pathway in cholangiocarcinoma
Cell Death & Differentiation (2025)
-
ATRA sensitized the response of hepatocellular carcinoma to Sorafenib by downregulation of p21-activated kinase 1
Cell Communication and Signaling (2023)