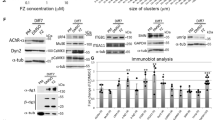
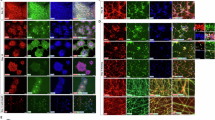
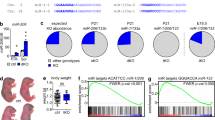
Abstract
Cellular retinoic acid-binding protein 1 (CRABP1) binds retinoic acid (RA) specifically in the cytoplasm with unclear functions. CRABP1 is highly and specifically expressed in spinal motor neurons (MNs). Clinical and pre-clinical data reveal a potential link between CRABP1 and MN diseases, including the amyotrophic lateral sclerosis (ALS). We established a sequenced MN-muscle co-differentiation system to engineer an in vitro functional 3D NMJ model for molecular studies and demonstrated that CRABP1 in MNs contributes to NMJ formation and maintenance. Consistently, Crabp1 knockout (CKO) mice exhibited an adult-onset ALS-like phenotype with progressively deteriorated NMJs, characterized with behavioral, EchoMRI, electrophysiological, histological, and immunohistochemical studies at 2–20-months old. Mechanistically, CRABP1 suppresses CaMKII activation to regulate neural Agrn expression and downstream muscle LRP4-MuSK signaling, thereby maintaining NMJ. A proof-of-concept was provided by specific re-expression of CRABP1 to rescue Agrn expression and the phenotype. This study identifies CRABP1-CaMKII-Agrn signaling as a physiological pre-synaptic regulator in the NMJ. This study also highlights a potential protective role of CRABP1 in the progression of NMJ deficits in MN diseases.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The data generated during this study are available upon reasonable request from the corresponding author.
References
Napoli JL. Retinoic acid: its biosynthesis and metabolism. Prog Nucleic Acid Res Mol Biol. 1999;63:139–88.
Persaud SD, Lin Y-W, Wu C-Y, Kagechika H, Wei L-N. Cellular retinoic acid binding protein I mediates rapid non-canonical activation of ERK1/2 by all-trans retinoic acid. Cell Signal. 2013;25:19–25.
Persaud SD, Park SW, Ishigami-Yuasa M, Koyano-Nakagawa N, Kagechika H, Wei LN. All trans-retinoic acid analogs promote cancer cell apoptosis through non-genomic Crabp1 mediating ERK1/2 phosphorylation. Sci Rep. 2016;6:22396.
Lin YL, Persaud SD, Nhieu J, Wei LN. Cellular retinoic acid-binding protein 1 modulates stem cell proliferation to affect learning and memory in male mice. Endocrinology. 2017;158:3004–14.
Park SW, Persaud SD, Ogokeh S, Meyers TA, Townsend DW, Wei LN. CRABP1 protects the heart from isoproterenol-induced acute and chronic remodeling. J Endocrinol. 2018;236:151–65.
Wu H, Xiong WC, Mei L. To build a synapse: signaling pathways in neuromuscular junction assembly. Development. 2010;137:1017–33.
Yumoto N, Kim N, Burden SJ. Lrp4 is a retrograde signal for presynaptic differentiation at neuromuscular synapses. Nature. 2012;489:438–42.
Li L, Xiong WC, Mei L. Neuromuscular junction formation, aging, and disorders. Annu Rev Physiol. 2018;80:159–88.
Taetzsch T, Valdez G. NMJ maintenance and repair in aging. Curr Opin Physiol. 2018;4:57–64.
Rizzo F, Nizzardo M, Vashisht S, Molteni E, Melzi V, Taiana M, et al. Key role of SMN/SYNCRIP and RNA-Motif 7 in spinal muscular atrophy: RNA-Seq and motif analysis of human motor neurons. Brain. 2019;142:276–94.
Maeda M, Harris AW, Kingham BF, Lumpkin CJ, Opdenaker LM, Mccahan SM, et al. Transcriptome profiling of spinal muscular atrophy motor neurons derived from mouse embryonic stem cells. PLoS One. 2014;9:e106818.
Lin YL, Lin YW, Nhieu J, Zhang X, Wei LN. Sonic hedgehog-gli1 signaling and cellular retinoic acid binding protein 1 gene regulation in motor neuron differentiation and diseases. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21114125.
Swindell WR, Bojanowski K, Kindy MS, Chau RMW, Ko D. GM604 regulates developmental neurogenesis pathways and the expression of genes associated with amyotrophic lateral sclerosis. Transl Neurodegener. 2018;7:1–24.
Jiang YM, Yamamoto M, Kobayashi Y, Yoshihara T, Liang Y, Terao S, et al. Gene expression profile of spinal motor neurons in sporadic amyotrophic lateral sclerosis. Ann Neurol. 2005;57:236–51.
Arellano-Ortiz AL, Salcedo-Vargas M, Vargas-Requena CL, López-Díaz JA, De la Mora-Covarrubias A, Silva-Espinoza JC, et al. DNA methylation of cellular retinoic acid-binding proteins in cervical cancer. Genet Epigenet. 2016;8:53–7.
Tanaka K, Imoto I, Inoue J, Kozaki K, Tsuda H, Shimada Y, et al. Frequent methylation-associated silencing of a candidate tumor-suppressor, CRABP1, in esophageal squamous-cell carcinoma. Oncogene. 2007;26:6456–68.
Südhof TC. Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron. 2013. https://doi.org/10.1016/j.neuron.2013.10.022.
Nhieu J, Lin YL, Wei LN. Noncanonical retinoic acid signaling. In: Methods in Enzymology. Academic Press Inc., 2020, pp 261–81.
Wook Park S, Nhieu J, Persaud SD, Miller MC, Xia Y, Lin YW, et al. A new regulatory mechanism for Raf kinase activation, retinoic acid-bound Crabp1. Sci Rep. 2019;9:10929.
Park SW, Nhieu J, Lin YW, Wei LN. All-trans retinoic acid attenuates isoproterenol-induced cardiac dysfunction through Crabp1 to dampen CaMKII activation. Eur J Pharm. 2019;858:172485.
Liu C, Hermann TE. Characterization of ionomycin as a calcium ionophore. J Biol Chem. 1978;253:5892–4.
Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu WL, et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci. 2017;20:1172–9.
Arnold AS, Gueye M, Guettier-Sigrist S, Courdier-Fruh I, Coupin G, Poindron P, et al. Reduced expression of nicotinic AChRs in myotubes from spinal muscular atrophy I patients. Lab Investig. 2004;84:1271–8.
Boido M, de Amicis E, Valsecchi V, Trevisan M, Ala U, Ruegg MA, et al. Increasing agrin function antagonizes muscle atrophy and motor impairment in spinal muscular atrophy. Front Cell Neurosci. 2018;12:17.
Bhattacharyya M, Karandur D, Kuriyan J. Structural insights into the regulation of Ca2+ /calmodulin-dependent protein kinase II (Camkii). Cold Spring Harb Perspect Biol. 2020;12:1–20.
Baltgalvis KA, Call JA, Nikas JB, Lowe DA. Effects of prednisolone on skeletal muscle contractility in mdx mice. Muscle Nerve. 2009;40:443–54.
Greising SM, Call JA, Lund TC, Blazar BR, Tolar J, Lowe DA. Skeletal muscle contractile function and neuromuscular performance in Zmpste24-/-mice, a murine model of human progeria. Age. 2012;34:805–19.
Garlich MW, Baltgalvis KA, Call JA, Dorsey LL, Lowe DA. Plantarflexion contracture in the mdx mouse. Am J Phys Med Rehabil. 2010;89:976–85.
Wang W, Qi B, Lv H, Wu F, Liu L, Wang W, et al. A new method of isolating spinal motor neurons from fetal mouse. J Neurosci Methods. 2017;288:57–61.
Sleigh JN, Burgess RW, Gillingwater TH, Cader MZ. Morphological analysis of neuromuscular junction development and degeneration in rodent lumbrical muscles. J Neurosci Methods. 2014;227:159–65.
Bolte S, Cordelières FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–32.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–67.
Théry M, Piel M. Adhesive micropatterns for cells: a microcontact printing protocol. Cold Spring Harb Protoc. 2009;4. https://doi.org/10.1101/pdb.prot5255.
Bettadapur A, Suh GC, Geisse NA, Wang ER, Hua C, Huber HA. et al. Prolonged culture of aligned skeletal myotubes on micromolded gelatin hydrogels. Sci Rep. 2016. https://doi.org/10.1038/srep28855.
Cammer .Measure intensity of a Z stack. http://microscopynotes.com/imagej/measure_Zstack_intensity/index.html. Accessed 15 Feb 2021.
Acknowledgements
We like to acknowledge University of Minnesota Viral Vector Core (VVC), Physiology Core, University Imaging Center (UIC) and staff support from Dr. Guillermo Marques and Dr. Mary Brown, and the Minnesota Nano Center (MNC) supported by the National Science Foundation through the National Nanotechnology Coordinated Infrastructure (NNCI) under Award Number ECCS-2025124. We would also like to thank Dr. Stanley Thayer for imaging equipment and analysis software. Figures depicting experimental procedures were Created with BioRender.com. This study was supported by DK54733 and DK60521, and University of Minnesota Medical School Dean’s Commitment to LNW, and F31DK123999 to JN. DJL and S-HO acknowledge support by the Minnesota Environment and Natural Resources Trust Fund as recommended by the Legislative-Citizen Commission on Minnesota Resources (LCCMR) and the Sanford P. Bordeau Chair in Electrical Engineering.
Author information
Authors and Affiliations
Contributions
YL designed and performed experiments and analyzed data; JN performed experiments and analyzed data; PL performed experiments and analyzed data; GL performed experiments and analyzed data; DJL generated reagents and materials; CW performed experiments. YWL performed experiments. S-HO provided reagents and materials and technical input DL provided reagents and materials and technical input. L-NW designed the experiments, analyzed the data, coordinated the study, and provided financial support. L-NW is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
All experimental procedures were conducted according to National Institutes of Health guidelines and were approved by the University of Minnesota Institutional Animal Care and Use Committee.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by L Greene.
Supplementary information
Rights and permissions
About this article
Cite this article
Lin, YL., Nhieu, J., Liu, PY. et al. CRABP1-CaMKII-Agrn regulates the maintenance of neuromuscular junction in spinal motor neuron. Cell Death Differ 29, 1744–1756 (2022). https://doi.org/10.1038/s41418-022-00959-4
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41418-022-00959-4
This article is cited by
-
Single-cell RNA transcriptome uncovers distinct developmental trajectories in the embryonic skeletal muscle of Daheng broiler and Tibetan chicken
BMC Genomics (2025)
-
Neuronal aging causes mislocalization of splicing proteins and unchecked cellular stress
Nature Neuroscience (2025)
-
The ARCCRABP1 neurons play a crucial role in the regulation of energy homeostasis
Nature Communications (2025)
-
CRABP1-complexes in exosome secretion
Cell Communication and Signaling (2024)
-
A novel 3D bilayer hydrogel tri-culture system for studying functional motor units
Cell & Bioscience (2023)