Abstract
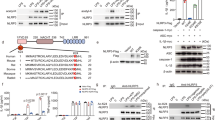
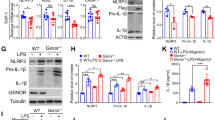
Subcellular machinery of NLRP3 is essential for inflammasome assembly and activation. However, the stepwise process and mechanistic basis of NLRP3 engagement with organelles remain unclear. Herein, we demonstrated glycogen synthase kinase 3β (GSK3β) as a molecular determinant for the spatiotemporal dynamics of NLRP3 inflammasome activation. Using live cell multispectral time-lapse tracking acquisition, we observed that upon stimuli NLRP3 was transiently associated with mitochondria and subsequently recruited to the Golgi network (TGN) where it was retained for inflammasome assembly. This occurred in relation to the temporal contact of mitochondria to Golgi apparatus. NLRP3 stimuli initiate GSK3β activation with subsequent binding to NLRP3, facilitating NLRP3 recruitment to mitochondria and transition to TGN. GSK3β activation also phosphorylates phosphatidylinositol 4-kinase 2 Α (PI4k2A) in TGN to promote sustained NLRP3 oligomerization. Our study has identified the interplay between GSK3β signaling and the organelles dynamics of NLRP3 required for inflammasome activation and opens new avenues for therapeutic intervention.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
All data are presented in the main text or supplementary materials. The expression plasmids reported in this manuscript are available at Addgene or upon request. The rest of the data supporting the present study are available from the corresponding author upon reasonable request.
References
Place DE, Kanneganti TD. Recent advances in inflammasome biology. Curr Opin Immunol. 2018;50:32–8.
Guo H, Callaway JB, Ting JP. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–87.
Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–206.
Cai X, Chen J, Xu H, Liu S, Jiang QX, Halfmann R, et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell. 2014;156:1207–22.
Chen J, Chen ZJ. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature. 2018;564:71–6.
Subramanian N, Natarajan K, Clatworthy MR, Wang Z, Germain RN. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell. 2013;153:348–61.
Anand PK, Malireddi RK, Kanneganti TD. Role of the nlrp3 inflammasome in microbial infection. Front Microbiol. 2011;2:12.
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–5.
Misawa T, Takahama M, Kozaki T, Lee H, Zou J, Saitoh T, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol. 2013;14:454–60.
Zhang Z, Meszaros G, He WT, Xu Y, de Fatima Magliarelli H, Mailly L, et al. Protein kinase D at the Golgi controls NLRP3 inflammasome activation. J Exp Med. 2017;214:2671–93.
Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39:311–23.
Valm AM, Cohen S, Legant WR, Melunis J, Hershberg U, Wait E, et al. Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature. 2017;546:162–7.
Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol. 2010;31:24–31.
Doble BW, Woodgett JR. GSK-3: Tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–86.
Woodgett JR. Judging a protein by more than its name: GSK-3. Sci STKE. 2001;2001:re12.
Ali A, Hoeflich KP, Woodgett JR. Glycogen synthase kinase-3: Properties, functions, and regulation. Chem Rev. 2001;101:2527–40.
Forde JE, Dale TC. Glycogen synthase kinase 3: A key regulator of cellular fate. Cell Mol Life Sci: CMLS. 2007;64:1930–44.
Glibo M, Serman A, Karin-Kujundzic V, Bekavac Vlatkovic I, Miskovic B, Vranic S, et al. The role of glycogen synthase kinase 3 (GSK3) in cancer with emphasis on ovarian cancer development and progression: A comprehensive review. Bosn J Basic Med Sci. 2021;21:5–18.
Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–5.
Broz P, Dixit VM. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–20.
Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001;7:1321–7.
Guo J, Wenk MR, Pellegrini L, Onofri F, Benfenati F, De Camilli P. Phosphatidylinositol 4-kinase type IIalpha is responsible for the phosphatidylinositol 4-kinase activity associated with synaptic vesicles. Proc Natl Acad Sci USA. 2003;100:3995–4000.
Robinson JW, Leshchyns’ka I, Farghaian H, Hughes WE, Sytnyk V, Neely GG, et al. PI4KIIalpha phosphorylation by GSK3 directs vesicular trafficking to lysosomes. Biochem J. 2014;464:145–56.
Balla A, Tuymetova G, Barshishat M, Geiszt M, Balla T. Characterization of type II phosphatidylinositol 4-kinase isoforms reveals association of the enzymes with endosomal vesicular compartments. J Biol Chem. 2002;277:20041–50.
Ouyang X, Ghani A, Malik A, Wilder T, Colegio OR, Flavell RA, et al. Adenosine is required for sustained inflammasome activation via the A(2)A receptor and the HIF-1alpha pathway. Nat Commun. 2013;4:2909.
Leng W, Ouyang X, Lei X, Wu M, Chen L, Wu Q, et al. The SGLT-2 inhibitor dapagliflozin has a therapeutic effect on atherosclerosis in diabetic ApoE(-/-) mice. Mediators Inflamm. 2016;2016:6305735.
Wiemerslage L, Lee D. Quantification of mitochondrial morphology in neurites of dopaminergic neurons using multiple parameters. J Neurosci Methods. 2016;262:56–65.
Bravo-Sagua R, Parra V, Ortiz-Sandoval C, Navarro-Marquez M, Rodriguez AE, Diaz-Valdivia N, et al. Caveolin-1 impairs PKA-DRP1-mediated remodelling of ER-mitochondria communication during the early phase of ER stress. Cell Death Differ. 2019;26:1195–212.
Zhao P, Han SN, Arumugam S, Yousaf MN, Qin Y, Jiang JX, et al. Digoxin improves steatohepatitis with differential involvement of liver cell subsets in mice through inhibition of PKM2 transactivation. American J Physiol Gastrointestinal Liver Physiol. 2019;317;G387–97.
Ouyang X, Han SN, Zhang JY, Dioletis E, Nemeth BT, Pacher P, et al. Digoxin suppresses Pyruvate Kinase M2-Promoted HIF-1alpha Transactivation in Steatohepatitis. Cell Metab. 2018;27:1156.
Acknowledgements
We thank Yale Liver Center Morphology Core/Molecular Core Facilities for the support of confocal microcopy, image processing and biological assay, Yale Proteomics Core Facility for the support of LC-MS/MS assay, Center for cellular and molecular imaging (CCMI) EM core facility at Yale medical school for EM processing. This study was supported by NIH UO1 grant 5U01AA026962-04 (to WZM and XO), by VA Merit award BX003259-05(to W.Z.M.), by Yale Liver Center Award NIH P30DK-034989 Morphology Core and Cellular /Molecular Core, and in part by Yale Liver Center Pilot Project Award (to XO) and NCI grant 5R01CA224023-03 (to RAF). The Yale Liver Center Core Facilities were funded by NIH by grant DK P30-034989.
Author information
Authors and Affiliations
Contributions
SA, RAF, WZM, and XO designed the research plan and interpreted the results. SA, YQ, ZL, SH, QL, and XO performed and analyzed the experiments. SA and. SB performed reversal experiments. SA, WZM, and XO wrote and WZM and XO edited the manuscript. XO supervised the project and gave final approval.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Our studies did not include human participants, human data, or human tissue. For the animal studies, experiments were performed according to both the Animal Care and Use Committee of Yale University and the Institutional Animal Ethics Committee/Institutional Animal Care and Use Committee (IACUC) of Army Medical University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by M. Piacentini
Supplementary information
Rights and permissions
About this article
Cite this article
Arumugam, S., Qin, Y., Liang, Z. et al. GSK3β mediates the spatiotemporal dynamics of NLRP3 inflammasome activation. Cell Death Differ 29, 2060–2069 (2022). https://doi.org/10.1038/s41418-022-00997-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41418-022-00997-y
This article is cited by
-
Updated insights into the molecular networks for NLRP3 inflammasome activation
Cellular & Molecular Immunology (2025)
-
Clustering of NLRP3 induced by membrane or protein scaffolds promotes inflammasome assembly
Nature Communications (2025)
-
ER-mitochondria association negatively affects wound healing by regulating NLRP3 activation
Cell Death & Disease (2024)
-
STAT3 promotes NLRP3 inflammasome activation by mediating NLRP3 mitochondrial translocation
Experimental & Molecular Medicine (2024)
-
Glycogen Synthase Kinase-3β, NLRP3 Inflammasome, and Alzheimer’s Disease
Current Medical Science (2023)