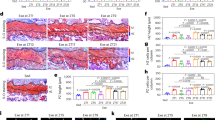
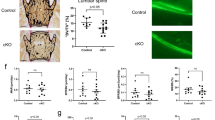
Abstract
Exercise in later life is important for bone health and delays the progression of osteoporotic bone loss. Osteocytes are the major bone cells responsible for transforming mechanical stimuli into cellular signals through their highly specialized lacunocanalicular networks (LCN). Osteocyte activity and LCN degenerate with aging, thus might impair the effectiveness of exercise on bone health; however, the underlying mechanism and clinical implications remain elusive. Herein, we showed that deletion of Sirt3 in osteocytes could impair the formation of osteocyte dendritic processes and inhibit bone gain in response to exercise in vivo. Mechanistic studies revealed that Sirt3 regulates E11/gp38 through the protein kinase A (PKA)/cAMP response element-binding protein (CREB) signaling pathway. Additionally, the Sirt3 activator honokiol enhanced the sensitivity of osteocytes to fluid shear stress in vitro, and intraperitoneal injection of honokiol reduced bone loss in aged mice in a dose-dependent manner. Collectively, Sirt3 in osteocytes regulates bone mass and mechanical responses through the regulation of E11/gp38. Therefore, targeting Sirt3 could be a novel therapeutic strategy to prevent age-related bone loss and augment the benefits of exercise on the senescent skeleton.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
All data relevant to the study are included in the article or uploaded as Supplementary Information.
References
Buenzli PR, Sims NA. Quantifying the osteocyte network in the human skeleton. Bone 2015;75:144–50.
Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–38.
Schneider P, Meier M, Wepf R, Müller R. Towards quantitative 3D imaging of the osteocyte lacuno-canalicular network. Bone 2010;47:848–58.
Bonewald L. Generation and function of osteocyte dendritic processes. J Musculoskelet Neuronal Interact. 2005;5:321.
Milovanovic P, Zimmermann EA, Hahn M, Djonic D, Püschel K, Djuric M, et al. Osteocytic canalicular networks: morphological implications for altered mechanosensitivity. ACS Nano. 2013;7:7542–51.
You L, Cowin SC, Schaffler MB, Weinbaum S. A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J Biomech. 2001;34:1375–86.
Nicolella DP, Moravits DE, Gale AM, Bonewald LF, Lankford J. Osteocyte lacunae tissue strain in cortical bone. J Biomech. 2006;39:1735–43.
Srinivasan S, Gross TS, Bain SD. Bone mechanotransduction may require augmentation in order to strengthen the senescent skeleton. Ageing Res Rev. 2012;11:353–60.
Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;7:CD000333.
Korpelainen R, Keinänen-Kiukaanniemi S, Heikkinen J, Väänänen K, Korpelainen J. Effect of impact exercise on bone mineral density in elderly women with low BMD: a population-based randomized controlled 30-month intervention. Osteoporos Int. 2006;17:109–18.
Busse B, Djonic D, Milovanovic P, Hahn M, Püschel K, Ritchie RO, et al. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell. 2010;9:1065–75.
Klein-Nulend J, Sterck J, Semeins C, Lips P, Joldersma M, Baart J, et al. Donor age and mechanosensitivity of human bone cells. Osteoporos Int. 2002;13:137–46.
Tiede-Lewis LM, Xie Y, Hulbert MA, Campos R, Dallas MR, Dusevich V, et al. Degeneration of the osteocyte network in the C57BL/6 mouse model of aging. Aging 2017;9:2190.
Masgras I, Cannino G, Ciscato F, Sanchez-Martin C, Darvishi FB, Scantamburlo F, et al. Tumor growth of neurofibromin-deficient cells is driven by decreased respiration and hampered by NAD+ and SIRT3. Cell Death Differ. 2022;29:1–13.
Kincaid B, Bossy-Wetzel E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front Aging Neurosci. 2013;5:48.
Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 2010;464:121–5.
Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D, et al. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci USA. 2011;108:14608–13.
Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell. 2011;44:177–90.
Gao J, Qin A, Liu D, Ruan R, Wang Q, Yuan J, et al. Endoplasmic reticulum mediates mitochondrial transfer within the osteocyte dendritic network. Sci Adv. 2019;5:eaaw7215.
Gao J, Feng Z, Wang X, Zeng M, Liu J, Han S, et al. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ. 2018;25:229–40.
Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL III, et al. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1α in skeletal muscle. Aging 2009;1:771.
Cheng A, Yang Y, Zhou Y, Maharana C, Lu D, Peng W, et al. Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metab. 2016;23:128–42.
Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiological Rev. 2009;89:121–45.
Delghandi MP, Johannessen M, Moens U. The cAMP signalling pathway activates CREB through PKA, p38 and MSK1 in NIH 3T3 cells. Cell Signal. 2005;17:1343–51.
Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age‐related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res. 2007;22:1197–207.
Okada S, Yoshida S, Ashrafi SH, Schraufnagel DE. The canalicular structure of compact bone in the rat at different ages. Microsc Microanalysis. 2002;8:104.
Zhang K, Barragan-Adjemian C, Ye L, Kotha S, Dallas M, Lu Y, et al. E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol. 2006;26:4539–52.
Prideaux M, Loveridge N, Pitsillides AA, Farquharson C. Extracellular matrix mineralization promotes E11/gp38 glycoprotein expression and drives osteocytic differentiation. PLoS ONE. 2012;7:e36786.
Staines KA, Javaheri B, Hohenstein P, Fleming R, Ikpegbu E, Unger E, et al. Hypomorphic conditional deletion of E11/Podoplanin reveals a role in osteocyte dendrite elongation. J Cell Physiol. 2017;232:3006–19.
Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22:119–41.
Scher MB, Vaquero A, Reinberg D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev. 2007;21:920–8.
Li Q, Cheng JCY, Jiang Q, Lee WYW. Role of sirtuins in bone biology: Potential implications for novel therapeutic strategies for osteoporosis. Aging Cell. 2021;20:e13301.
Dobson PF, Dennis EP, Hipps D, Reeve A, Laude A, Bradshaw C, et al. Mitochondrial dysfunction impairs osteogenesis, increases osteoclast activity, and accelerates age related bone loss. Sci Rep. 2020;10:1–14.
Figueiredo PA, Powers SK, Ferreira RM, Amado F, Appell HJ, Duarte JA. Impact of lifelong sedentary behavior on mitochondrial function of mice skeletal muscle. J Gerontol Ser A Biomed Sci Med Sci. 2009;64:927–39.
Kim JM, Choi JS, Kim YH, Jin SH, Lim S, Jang HJ, et al. An activator of the cAMP/PKA/CREB pathway promotes osteogenesis from human mesenchymal stem cells. J Cell Physiol. 2013;228:617–26.
Long F, Schipani E, Asahara H, Kronenberg H, Montminy M. The CREB family of activators is required for endochondral bone development. Development 2001;128:541–50.
Sato K, Suematsu A, Nakashima T, Takemoto-Kimura S, Aoki K, Morishita Y, et al. Regulation of osteoclast differentiation and function by the CaMK-CREB pathway. Nat Med. 2006;12:1410–6.
Wu Z, Huang X, Feng Y, Handschin C, Feng Y, Gullicksen PS, et al. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1α transcription and mitochondrial biogenesis in muscle cells. Proc Natl Acad Sci USA. 2006;103:14379–84.
Schwer B, North BJ, Frye RA, Ott M, Verdin E. The human silent information regulator (Sir) 2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide–dependent deacetylase. J Cell Biol. 2002;158:647–57.
Kim H-S, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17:41–52.
Matsui N, Takahashi K, Takeichi M, Kuroshita T, Noguchi K, Yamazaki K, et al. Magnolol and honokiol prevent learning and memory impairment and cholinergic deficit in SAMP8 mice. Brain Res. 2009;1305:108–17.
Liou K-T, Shen Y-C, Chen C-F, Tsao C-M, Tsai S-K. Honokiol protects rat brain from focal cerebral ischemia–reperfusion injury by inhibiting neutrophil infiltration and reactive oxygen species production. Brain Res. 2003;992:159–66.
Pillai VB, Samant S, Sundaresan NR, Raghuraman H, Kim G, Bonner MY, et al. Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial Sirt3. Nat Commun. 2015;6:1–16.
Zhang L, Wang X. Hydrophobic ionic liquid‐based ultrasound‐assisted extraction of magnolol and honokiol from cortex Magnoliae officinalis. J Sep Sci. 2010;33:2035–8.
Bause AS, Haigis MC. SIRT3 regulation of mitochondrial oxidative stress. Exp Gerontol. 2013;48:634–9.
Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–97.
Galliera E, Massaccesi L, Banfi G, De Vecchi E, Ragone V, Corsi Romanelli MM, et al. Effect of oxidative stress on bone remodeling in periprosthetic osteolysis. Clin Rev Bone Miner Metab. 2021;19:14–23.
Lu Y, Xie Y, Zhang S, Dusevich V, Bonewald L, Feng J. DMP1-targeted Cre expression in odontoblasts and osteocytes. J Dent Res. 2007;86:320–5.
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J Bone Min Res. 2010;25:1468–86.
Cheuk KY, Wang XF, Wang J, Zhang Z, Yu FWP, Tam EMS, et al. Sexual dimorphism in cortical and trabecular bone microstructure appears during puberty in Chinese children. J Bone Miner Res. 2018;33:1948–55.
Crowe AR, Yue W. Semi-quantitative determination of protein expression using immunohistochemistry staining and analysis: an integrated protocol. Bio Protoc. 2019;9:e3465.
Chen H, Zhang J, Wang Y, Cheuk KY, Hung AL, Lam TP, et al. Abnormal lacuno‐canalicular network and negative correlation between serum osteocalcin and Cobb angle indicate abnormal osteocyte function in adolescent idiopathic scoliosis. FASEB J. 2019;33:13882–92.
Ren Y, Lin S, Jing Y, Dechow P, Feng JQ. A novel way to statistically analyze morphologic changes in Dmp1-null osteocytes. Connect Tissue Res. 2014;55:129–33.
Zhang J, Chen H, Leung RK, Choy KW, Lam TP, Ng BK, et al. Aberrant miR‐145–5p/β‐catenin signal impairs osteocyte function in adolescent idiopathic scoliosis. FASEB J. 2018;32:6537–49.
Kelly NH, Schimenti JC, Ross FP, van der Meulen MC. A method for isolating high quality RNA from mouse cortical and cancellous bone. Bone 2014;68:1–5.
Stern AR, Stern MM, Van Dyke ME, Jähn K, Prideaux M, Bonewald LF. Isolation and culture of primary osteocytes from the long bones of skeletally mature and aged mice. Biotechniques 2012;52:361–73.
Stegen S, van Gastel N, Eelen G, Ghesquière B, D’Anna F, Thienpont B, et al. HIF-1α promotes glutamine-mediated redox homeostasis and glycogen-dependent bioenergetics to support postimplantation bone cell survival. Cell Metab. 2016;23:265–79.
Wang Y, Zhao X, Lotz M, Terkeltaub R, Liu‐Bryan R. Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator–activated receptor γ coactivator 1α. Arthritis Rheumatol. 2015;67:2141–53.
Acknowledgements
We thank Johan Auwerx for providing the Sirt3 floxed mice and Lynda Bonewald for providing the MLO-Y4 cell lines. We thank Dr. Cao Qian (visiting scholar from Nanjing Medical University) for helping with the animal breeding.
Funding
This work was substantially supported by Start-up grant from Chinese University of Hong Kong (Ref Nos. 4930991 and 4930992), and partly supported by the General Research Fund (Ref Nos. 14163517, 14120818 and 14104620) and Research Matching Grant Scheme, University Grants Committee, HKSAR; Health and Medical Research Fund, The Food and Health Bureau, HKSAR (Ref No. 06170546); and Area of Excellence, University Grants Committee, HKSAR (AoE/M-402/20), 2020 The American Society for Bone and Mineral Research (ASBMR) Rising Star Award, Major Project of Natural Science Foundation of China (Ref No. 81991514), Young Scientists Fund of the Natural Science Foundation of China (Ref No. 82202755), and Young Scientists Fund of the Natural Science Foundation of Jiangsu Province, China (Ref No. BK20220183).
Author information
Authors and Affiliations
Contributions
WYWL and QQL conceived the project. WYWL and QQL designed the study. QQL conducted most assays and acquired and analyzed data. RLW, ZZ, HXW, XML, and JJZ participated in some experiments. APKK, XYT, HFC, ACKC, and QJ provided technical and material support. QQL drafted the manuscript. QJ, JCYC and WYWL revised the manuscript. All authors approved the final version of the manuscript. QQL and WYWL take responsibility for the integrity of the data analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Our studies did not include human participants or human tissue. Animal studies were approved by the Animal Experimentation Ethics Committee of The Chinese University of Hong Kong (ethical approval number 19-159-MIS).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by: M. Piacentini
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Q., Wang, R., Zhang, Z. et al. Sirt3 mediates the benefits of exercise on bone in aged mice. Cell Death Differ 30, 152–167 (2023). https://doi.org/10.1038/s41418-022-01053-5
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41418-022-01053-5
This article is cited by
-
Enhanced SIRT3 expression restores mitochondrial quality control mechanism to reverse osteogenic impairment in type 2 diabetes mellitus
Bone Research (2025)
-
Age-related alveolar bone maladaptation in adult orthodontics: finding new ways out
International Journal of Oral Science (2024)
-
Bone-targeting engineered small extracellular vesicles carrying anti-miR-6359-CGGGAGC prevent valproic acid-induced bone loss
Signal Transduction and Targeted Therapy (2024)