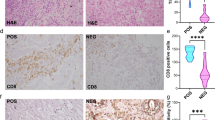
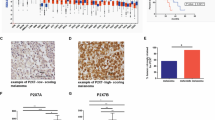
Abstract
Uncontrolled inflammatory response arising from the tumor microenvironment (TME) significantly contributes to cancer progression, prompting an investigation and careful evaluation of counter-regulatory mechanisms. We identified a trimeric complex at the mitochondria-associated membranes (MAMs), in which the purinergic P2X7 receptor - NLRP3 inflammasome liaison is fine-tuned by the tumor suppressor PML. PML downregulation drives an exacerbated immune response due to a loss of P2X7R-NLRP3 restraint that boosts tumor growth. PML mislocalization from MAMs elicits an uncontrolled NLRP3 activation, and consequent cytokines blast fueling cancer and worsening the tumor prognosis in different human cancers. New mechanistic insights are provided for the PML-P2X7R-NLRP3 axis to govern the TME in human carcinogenesis, fostering new targeted therapeutic approaches.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The data analyzed during this study are included in this article and the supplemental data files. Additional supporting data are available from the corresponding authors upon reasonable request.
References
Pinton P, Giorgi C, Pandolfi PP. The role of PML in the control of apoptotic cell fate: a new key player at ER-mitochondria sites. Cell Death Differ. 2011;18:1450–6.
Giorgi C, Ito K, Lin HK, Santangelo C, Wieckowski MR, Lebiedzinska M, et al. PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Science. 2010;330:1247–51.
Missiroli S, Bonora M, Patergnani S, Poletti F, Perrone M, Gafa R, et al. PML at mitochondria-associated membranes is critical for the repression of autophagy and cancer development. Cell Rep. 2016;16:2415–27.
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–5.
Missiroli S, Patergnani S, Caroccia N, Pedriali G, Perrone M, Previati M, et al. Mitochondria-associated membranes (MAMs) and inflammation. Cell Death Dis. 2018;9:329.
Karki R, Kanneganti TD. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat Rev Cancer. 2019;19:197–214.
Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411.
Karmakar M, Katsnelson MA, Dubyak GR, Pearlman E. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1beta secretion in response to ATP. Nat Commun. 2016;7:10555.
Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 receptor in infection and inflammation. Immunity. 2017;47:15–31.
Franceschini A, Capece M, Chiozzi P, Falzoni S, Sanz JM, Sarti AC, et al. The P2X7 receptor directly interacts with the NLRP3 inflammasome scaffold protein. FASEB J. 2015;29:2450–61.
Adinolfi E, Giuliani AL, De Marchi E, Pegoraro A, Orioli E, Di Virgilio F. The P2X7 receptor: a main player in inflammation. Biochem Pharmacol. 2018;151:234–44.
Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328.
Tweedell RE, Malireddi RKS, Kanneganti TD. A comprehensive guide to studying inflammasome activation and cell death. Nat Protoc. 2020;15:3284–333.
Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–55.
Adinolfi E, Raffaghello L, Giuliani AL, Cavazzini L, Capece M, Chiozzi P, et al. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 2012;72:2957–69.
Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–20.
Missiroli S, Perrone M, Boncompagni C, Borghi C, Campagnaro A, Marchetti F, et al. Targeting the NLRP3 inflammasome as a new therapeutic option for overcoming cancer. Cancers. 2021;13:2297.
Mantovani A, Barajon I, Garlanda C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol Rev. 2018;281:57–61.
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99.
Guerriero JL. Macrophages: the road less traveled, changing anticancer therapy. Trends Mol Med. 2018;24:472–89.
Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12:76.
Arwert EN, Harney AS, Entenberg D, Wang Y, Sahai E, Pollard JW, et al. A unidirectional transition from migratory to perivascular macrophage is required for tumor cell intravasation. Cell Rep. 2018;23:1239–48.
Franklin RA, Li MO. Ontogeny of tumor-associated macrophages and its implication in cancer regulation. Trends Cancer. 2016;2:20–34.
Lo YH, Huang YW, Wu YH, Tsai CS, Lin YC, Mo ST, et al. Selective inhibition of the NLRP3 inflammasome by targeting to promyelocytic leukemia protein in mouse and human. Blood. 2013;121:3185–94.
Dowling JK, Becker CE, Bourke NM, Corr SC, Connolly DJ, Quinn SR, et al. Promyelocytic leukemia protein interacts with the apoptosis-associated speck-like protein to limit inflammasome activation. J Biol Chem. 2014;289:6429–37.
Liu CY, Xu JY, Shi XY, Huang W, Ruan TY, Xie P, et al. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Investig. 2013;93:844–54.
Zhang Q, Wang H, Mao C, Sun M, Dominah G, Chen L, et al. Fatty acid oxidation contributes to IL-1beta secretion in M2 macrophages and promotes macrophage-mediated tumor cell migration. Mol Immunol. 2018;94:27–35.
Wang YT, Chen J, Chang CW, Jen J, Huang TY, Chen CM, et al. Ubiquitination of tumor suppressor PML regulates prometastatic and immunosuppressive tumor microenvironment. J Clin Investig. 2017;127:2982–97.
Scherer M, Stamminger T. Emerging role of PML nuclear bodies in innate immune signaling. J Virol. 2016;90:5850–4.
Lin S, Mei X. Role of NLRP3 inflammasomes in neuroinflammation diseases. Eur Neurol. 2020;83:576–80.
Kuchay S, Giorgi C, Simoneschi D, Pagan J, Missiroli S, Saraf A, et al. PTEN counteracts FBXL2 to promote IP3R3- and Ca(2+)-mediated apoptosis limiting tumour growth. Nature. 2017;546:554–8.
Ying W, Cheruku PS, Bazer FW, Safe SH, Zhou B. Investigation of macrophage polarization using bone marrow derived macrophages. J Vis Exp. 2013;50323.
Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008;Chapter 14:Unit 14.1.
Riedhammer C, Halbritter D, Weissert R. Peripheral blood mononuclear cells: isolation, freezing, thawing, and culture. Methods Mol Biol. 2016;1304:53–61.
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–308.
Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990;265:7248–56.
Wieckowski MR, Giorgi C, Lebiedzinska M, Duszynski J, Pinton P. Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat Protoc. 2009;4:1582–90.
Marchi S, Corricelli M, Trapani E, Bravi L, Pittaro A, Delle Monache S, et al. Defective autophagy is a key feature of cerebral cavernous malformations. EMBO Mol Med. 2015;7:1403–17.
Acknowledgements
The authors thank C. Bosi, F. Poletti, A.C. Sarti, and S. Falzoni for their contribution to this study. PP is grateful to C. degli Scrovegni for her continuous support.
Funding
The Signal Transduction Laboratory is supported by the Italian Association for Cancer Research (IG-23670 to PP, IG-19803 to CG and IG 22883 to FDV), A-ROSE, Progetti di Rilevante Interesse Nazionale (PRIN2017E5L5P3 to PP and PRIN20177E9EPY to CG), the Italian Ministry of Health (GR-2019-12369646 to SM), the European Research Council (853057-InflaPML to CG) and local funds from the University of Ferrara to CG and PP. MRW was supported by the National Science Centre, Poland (UMO-2018/29/B/NZ1/00589). MLA was funded by a Polish National Science Centre grant (UMO-2015/17/D/NZ1/00030). MP is supported by an AIRC research fellowship (ID: 26665).
Author information
Authors and Affiliations
Contributions
CG conceived the study. SM performed most of the experiments and prepared the figures. MP and SMa performed the proximity ligation assay studies. MP generated the CRISPR knockout clones. CG directed and coordinated the study, oversaw all the experiments, and wrote the manuscript. PP supervised the study and oversaw the experiments. F.D.V. collaborated in experiments related to P2X7R. GM, MRW, and MLA performed the co-immunoprecipitation experiments. RG, GL and LA performed the immunohistochemical analysis. LA helped in writing the manuscript. SM and MP provided advice for the in vivo experiments. FN performed the flow cytometry experiments. MB and KI performed the bone marrow replacement experiment. CB and BV helped with some experiments. SM performed the Western blot, ELISA, and subcellular fractionation studies. FK performed the SPR studies. FF, AB, and RG helped in the patient’s cohorts selection. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Procedures involving animals and their care were in conformity with institutional guidelines, and the Animal Ethics Committee approved all experimental protocols (authorization nos. 481/2017-PR and CBCC2.N.BH4 approved by the Italian Ministry of Health). The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the University Hospital of Ferrara (CE-AVEC: 1016/2020/Oss/UniFe).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by P Salomoni
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Missiroli, S., Perrone, M., Gafà, R. et al. PML at mitochondria-associated membranes governs a trimeric complex with NLRP3 and P2X7R that modulates the tumor immune microenvironment. Cell Death Differ 30, 429–441 (2023). https://doi.org/10.1038/s41418-022-01095-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-022-01095-9
This article is cited by
-
Organelle stress in NLRP3 inflammasome: a central mediator of neurodegenerative diseases
Molecular Neurodegeneration (2026)
-
The importance of mitochondria and mitochondrial calcium signaling in health and disease: an updated outlook on inflammation
Journal of Translational Medicine (2026)
-
NADPH acts as an endogenous P2X7 receptor modulator to gate neuroinflammatory responses of microglia
Acta Pharmacologica Sinica (2026)
-
Radiation meets inflammation: NLRP3 inflammasome at the core of radiation-induced cardiac injury
Journal of Translational Medicine (2025)
-
Review: Progress of the NLRP3 inflammasome in tumours and perspectives for cholangiocarcinoma
Cell Communication and Signaling (2025)