Abstract
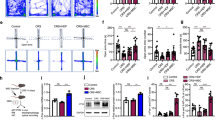
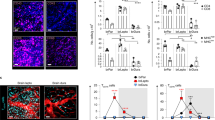
The dura sinus-resident immune cells can influence the process of central neural system (CNS) diseases by communicating with central nerve cells. In clinical, Tregs are also frequently impaired in depression. However, the significance of this relationship remains unknown. In the present study, we found a significant increase in dural Treg populations in mouse models of depression, whereas depleting them by neutralizing antibodies injection could exacerbate depressive phenotypes. Through RNA sequencing, we identified that the antidepressant effects of dural Tregs are at least in part through the production of amphiregulin, increasing the expression of its receptor EGFR in medial prefrontal cortex (mPFC) pyramidal neurons. Furthermore, dural Tregs expressed high levels of ST2, and their expansion in depressed mice depended on astrocyte-derived IL33 secretion. Our study shows that dural Treg signaling can be enhanced by treatment with fluoxetine, highlighting that dural Tregs can be utilized as a potential target cell in major depressive disorder (MDD).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The datasets used in the current study are available on the Sequence Read Archive (SRA) repository (No. PRJNA1177115; https://submit.ncbi.nlm.nih.gov/subs/sra/). Additional data are available from authors upon request.
References
Ren HM, Kolawole EM, Ren M, Jin G, Netherby-Winslow CS, Wade Q, et al. IL-21 from high-affinity CD4 T cells drives differentiation of brain-resident CD8 T cells during persistent viral infection. Sci Immunol. 2020;5:eabb5590.
Alves de Lima K, Rustenhoven J, Da Mesquita S, Wall M, Salvador AF, Smirnov I, et al. Meningeal gammadelta T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat Immunol. 2020;21:1421–29.
Ribeiro M, Brigas HC, Temido-Ferreira M, Pousinha PA, Regen T, Santa C, et al. Meningeal γδ T cell–derived IL-17 controls synaptic plasticity and short-term memory. Sci Immunol. 2019;4:eaay5199.
Gate D, Tapp E, Leventhal O, Shahid M, Nonninger TJ, Yang AC, et al. CD4(+) T cells contribute to neurodegeneration in Lewy body dementia. Science. 2021;374:868–74.
Karikari AA, McFleder RL, Ribechini E, Blum R, Bruttel V, Knorr S, et al. Neurodegeneration by alpha-synuclein-specific T cells in AAV-A53T-alpha-synuclein Parkinson’s disease mice. Brain Behav Immun. 2022;101:194–210.
Sanmarco LM, Wheeler MA, Gutiérrez-Vázquez C, Polonio CM, Linnerbauer M, Pinho-Ribeiro FA, et al. Gut-licensed IFNgamma(+) NK cells drive LAMP1(+)TRAIL(+) anti-inflammatory astrocytes. Nature. 2021;590:473–79.
Lynall ME, Turner L, Bhatti J, Cavanagh J, de Boer P, Mondelli V, et al. Peripheral blood cell-stratified subgroups of inflamed depression. Biol Psychiatry. 2020;88:185–96.
Beurel E, Harrington LE, Jope RS. Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol Psychiatry. 2013;73:622–30.
Medina-Rodriguez EM, Madorma D, O’Connor G, Mason BL, Han D, Deo SK, et al. Identification of a signaling mechanism by which the microbiome regulates Th17 cell-mediated depressive-like behaviors in mice. Am J Psychiatry. 2020;177:974–90.
Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 2019;565:246–50.
Shi L, Sun Z, Su W, Xu F, Xie D, Zhang Q, et al. Treg cell-derived osteopontin promotes microglia-mediated white matter repair after ischemic stroke. Immunity. 2021;54:1527–42.e1528.
Westfall S, Caracci F, Zhao D, Wu QL, Frolinger T, Simon J, et al. Microbiota metabolites modulate the T helper 17 to regulatory T cell (Th17/Treg) imbalance promoting resilience to stress-induced anxiety- and depressive-like behaviors. Brain Behav Immun. 2021;91:350–68.
Westfall S, Caracci F, Estill M, Frolinger T, Shen L, Pasinetti GM. Chronic stress-induced depression and anxiety priming modulated by gut-brain-axis immunity. Front Immunol. 2021;12:670500.
Song L, Sen S, Sun Y, Zhou J, Mo L, He Y. Ketamine inhalation ameliorates ovalbumin-induced murine asthma by suppressing the epithelial-mesenchymal transition. Med Sci Monit. 2016;22:2471–83.
Wiese T, Dennstädt F, Hollmann C, Stonawski S, Wurst C, Fink J, et al. Inhibition of acid sphingomyelinase increases regulatory T cells in humans. Brain Commun. 2021;3:fcab020.
Sacramento PM, Monteiro C, Dias ASO, Kasahara TM, Ferreira TB, Hygino J, et al. Serotonin decreases the production of Th1/Th17 cytokines and elevates the frequency of regulatory CD4(+) T-cell subsets in multiple sclerosis patients. Eur J Immunol. 2018;48:1376–88.
Rustenhoven J, Drieu A, Mamuladze T, de Lima KA, Dykstra T, Wall M, et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell. 2021;184:1000–16.e1027.
Grosse L, Carvalho LA, Birkenhager TK, Hoogendijk WJ, Kushner SA, Drexhage HA, et al. Circulating cytotoxic T cells and natural killer cells as potential predictors for antidepressant response in melancholic depression. Restoration of T regulatory cell populations after antidepressant therapy. Psychopharmacology. 2016;233:1679–88.
Mohd Ashari NS, Mohamed Sanusi SNF, Mohd Yasin MA, Che Hussin CM, Wong KK, Shafei MN. Major depressive disorder patients on antidepressant treatments display higher number of regulatory T cells. Malays J Pathol. 2019;41:169–76.
Niu C, Yu J, Zou T, Lu Y, Deng L, Yun H, et al. Identification of hematopoietic stem cells residing in the meninges of adult mice at steady state. Cell Rep. 2022;41:111592.
Faustino LD, Griffith JW, Rahimi RA, Nepal K, Hamilos DL, Cho JL, et al. Interleukin-33 activates regulatory T cells to suppress innate gammadelta T cell responses in the lung. Nat Immunol. 2020;21:1371–83.
Pandolfo G, Genovese G, Casciaro M, Muscatello MRA, Bruno A, Pioggia G, et al. IL-33 in mental disorders. Medicina. 2021;57:315.
Durkee C, Kofuji P, Navarrete M, Araque A. Astrocyte and neuron cooperation in long-term depression. Trends Neurosci. 2021;44:837–48.
Liu J, Mo JW, Wang X, An Z, Zhang S, Zhang CY, et al. Astrocyte dysfunction drives abnormal resting-state functional connectivity in depression. Sci Adv. 2022;8:eabo2098.
Yirmiya R, Rimmerman N, Reshef R. Depression as a microglial disease. Trends Neurosci. 2015;38:637–58.
Badr M, McFleder RL, Wu J, Knorr S, Koprich JB, Hünig T, et al. Expansion of regulatory T cells by CD28 superagonistic antibodies attenuates neurodegeneration in A53T-alpha-synuclein Parkinson’s disease mice. J Neuroinflammation. 2022;19:319.
Romano R, Bucci C. Role of EGFR in the nervous system. Cells. 2020;9:1887.
Lin S, Huang L, Luo ZC, Li X, Jin SY, Du ZJ, et al. The ATP level in the medial prefrontal cortex regulates depressive-like behavior via the medial prefrontal cortex-lateral habenula pathway. Biol Psychiatry. 2022;92:179–92.
Patent: Ji MJ, l. R, Ni YY, inventors; Nanjing ZHISHI Law Firm, assignee. Methods for preparation and application of polypeptide. CN patent CN112707959B. 2022.
Sharma A, Rudra D. Emerging functions of regulatory T cells in tissue homeostasis. Front Immunol. 2018;9:883.
Vainchtein ID, Chin G, Cho FS, Kelley KW, Miller JG, Chien EC, et al. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science. 2018;359:1269–73.
Wang Y, Fu WY, Cheung K, Hung KW, Chen C, Geng H, et al. Astrocyte-secreted IL-33 mediates homeostatic synaptic plasticity in the adult hippocampus. Proc Natl Acad Sci USA. 2021;118:e2020810118.
Liu Y, Song N, Yao H, Jiang S, Wang Y, Zheng Y, et al. β-Arrestin2-biased Drd2 agonist UNC9995 alleviates astrocyte inflammatory injury via interaction between β-arrestin2 and STAT3 in mouse model of depression. J Neuroinflammation. 2022;19:240.
Guo S, Luo Y. Brain Foxp3+ regulatory T cells can be expanded by Interleukin-33 in mouse ischemic stroke. Int Immunopharmacol. 2020;81:106027.
Zhan L, Zheng L, Hosoi T, Okuma Y, Nomura Y. Stress-induced neuroprotective effects of epiregulin and amphiregulin. PLoS One. 2015;10:e0118280.
Steponaitis G, Kazlauskas A, Skiriute D, Vaitkiene P, Skauminas K, Tamasauskas A. Significance of amphiregulin (AREG) for the outcome of low and high grade astrocytoma patients. J Cancer. 2019;10:1479–88.
Yang J, Cheng X, Qi J, Xie B, Zhao X, Zheng K, et al. EGF enhances oligodendrogenesis from glial progenitor cells. Front Mol Neurosci. 2017;10:106.
Liu B, Neufeld AH. Activation of epidermal growth factor receptors directs astrocytes to organize in a network surrounding axons in the developing rat optic nerve. Dev Biol. 2004;273:297–307.
Hu Q, Zhang L, Wen J, Wang S, Li M, Feng R, et al. The EGF receptor-sox2-EGF receptor feedback loop positively regulates the self-renewal of neural precursor cells. Stem Cells. 2010;28:279–86.
Sarantis P, Trifylli EM, Koustas E, Papavassiliou KA, Karamouzis MV, Papavassiliou AG. Immune microenvironment and immunotherapeutic management in virus-associated digestive system tumors. Int J Mol Sci. 2022;23:13612.
Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22.
Xu Z, Zhang X, Chang H, Kong Y, Ni Y, Liu R, et al. Rescue of maternal immune activation-induced behavioral abnormalities in adult mouse offspring by pathogen-activated maternal Treg cells. Nat Neurosci. 2021;24:818–30.
Baruch K, Rosenzweig N, Kertser A, Deczkowska A, Sharif AM, Spinrad A, et al. Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer’s disease pathology. Nat Commun. 2015;6:7967.
Calahorra L, Camacho-Toledano C, Serrano-Regal MP, Ortega MC, Clemente D. Regulatory cells in multiple sclerosis: from blood to brain. Biomedicines. 2022;10:335.
Ellul P, Mariotti-Ferrandiz E, Leboyer M, Klatzmann D. Regulatory T cells as supporters of psychoimmune resilience: toward immunotherapy of major depressive disorder. Front Neurol. 2018;9:167.
Whibley N, Tucci A, Powrie F. Regulatory T cell adaptation in the intestine and skin. Nat Immunol. 2019;20:386–96.
Pröbstel AK, Zhou X, Baumann R, Wischnewski S, Kutza M, Rojas OL, et al. Gut microbiota-specific IgA+ B cells traffic to the CNS in active multiple sclerosis. Sci Immunol. 2020;5:eabc7191.
Fitzpatrick Z, Frazer G, Ferro A, Clare S, Bouladoux N, Ferdinand J, et al. Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature. 2020;587:472–76.
McGuinness AJ, Davis JA, Dawson SL, Loughman A, Collier F, O’Hely M, et al. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol Psychiatry. 2022;27:1920–35.
Gao K, Mu CL, Farzi A, Zhu WY. Tryptophan metabolism: a link between the gut microbiota and brain. Adv Nutr. 2020;11:709–23.
Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–12.
Foley ÉM, Parkinson JT, Mitchell RE, Turner L, Khandaker GM. Peripheral blood cellular immunophenotype in depression: a systematic review and meta-analysis. Mol Psychiatry 2023;28:1004–19.
Li Z, Vidjro OE, Guo G, Du Y, Zhou Y, Xie Q, et al. NLRP3 deficiency decreases alcohol intake controlling anxiety-like behavior via modification of glutamatergic transmission in corticostriatal circuits. J Neuroinflammation. 2022;19:308.
Funding
National Key R&D Program of China (No. 2021ZD0202903). National Natural Science Foundation of China (Nos. 82373851, 81922066, 82173797, 81991523).
Author information
Authors and Affiliations
Contributions
Experimental operation: Hang Yao, Yuepin Wang, You Xue, Siyuan Jiang, Xin Sun. Data acquisition: Hang Yao, Yang Liu. Figure drafting: Hang Yao, Yang Liu. Manuscript writing: Hang Yao, Yang Liu. Single-cell sequencing analysis guidance: Zhipeng Xu, Minjun Ji. Technical supporting: Jianhua Ding. Study designing and manuscript revising: Ming Lu, Gang Hu.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, H., Liu, Y., Wang, Y. et al. Dural Tregs driven by astrocytic IL-33 mitigate depression through the EGFR signals in mPFC neurons. Cell Death Differ 32, 926–943 (2025). https://doi.org/10.1038/s41418-024-01421-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-024-01421-3