Abstract
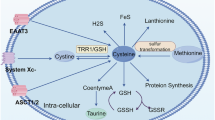
Disulfidptosis is a recently identified form of cell death characterized by the aberrant accumulation of cellular disulfides. This process primarily occurs in glucose-starved cells expressing higher levels of SLC7A11 and has been proposed as a therapeutic strategy for cancers with hyperactive SCL7A11. However, the potential for inducing disulfidptosis through other mechanisms in cancers remains unclear. Here, we found that inhibiting thioredoxin reductase 1 (TrxR1), a key enzyme in the thioredoxin system, induces disulfidptosis in glioblastoma (GBM) cells. TrxR1 expression is elevated in GBM with activated transcriptional coactivator with PDZ-binding motif (TAZ) and correlates with poor prognosis. TrxR1 inhibitors induced GBM cell death that can be rescued by disulfide reducers but not by ROS scavengers or inhibitors of apoptosis, ferroptosis, or necroptosis. Glucose-starved cells, but not those deprived of oxygen or glutamine, increased TrxR1 expression in an NRF2-dependent manner and were more sensitive to TrxR1 inhibition-induced cell death. The dying cells initially exhibited highly dynamic lamellipodia, followed by actin cytoskeleton collapse. This process involved the accumulation of cytosolic peroxisomes and micropinocytic caveolae, as well as small gaps in the plasma membrane. Depletion of the WAVE complex component NCKAP1 partially rescued the cells, whereas Rac inhibition enhanced cell death. In an orthotopic xenograft GBM mouse model, TrxR1 depletion inhibited tumor growth and improved survival. Furthermore, cells undergoing TrxR1 inhibition exhibited features of immunogenic cell death. Therefore, this study suggests the potential of targeting TrxR1 as a therapeutic strategy in GBM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The RNA-seq datasets from this study have been deposited to the Gene Expression Omnibus with the accession # GSE282324, GSE282325, and GSE282334.
References
Liu X, Nie L, Zhang Y, Yan Y, Wang C, Colic M, et al. Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis. Nat Cell Biol. 2023;25:404–14.
Liu X, Zhuang L, Gan B. Disulfidptosis: disulfide stress-induced cell death. Trends Cell Biol. 2024;34:327–37.
Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109:93–108.
Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 2020;20:12–25.
Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–73.
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110.
Bhat KP, Salazar KL, Balasubramaniyan V, Wani K, Heathcock L, Hollingsworth F, et al. The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes Dev. 2011;25:2594–609.
Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463:318–25.
Zheng Y, Pan D. The Hippo signaling pathway in development and disease. Dev Cell. 2019;50:264–82.
Ma S, Meng Z, Chen R, Guan KL. The Hippo pathway: biology and pathophysiology. Annu Rev Biochem. 2019;88:577–604.
Baroja I, Kyriakidis NC, Halder G, Moya IM. Expected and unexpected effects after systemic inhibition of Hippo transcriptional output in cancer. Nat Commun. 2024;15:2700.
Lee CK, Jeong SH, Jang C, Bae H, Kim YH, Park I, et al. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science. 2019;363:644–9.
Piccolo S, Panciera T, Contessotto P, Cordenonsi M. YAP/TAZ as master regulators in cancer: modulation, function and therapeutic approaches. Nat Cancer. 2023;4:9–26.
Wu K, El Zowalaty AE, Sayin VI, Papagiannakopoulos T. The pleiotropic functions of reactive oxygen species in cancer. Nat Cancer. 2024;5:384–99.
Reczek CR, Chandel NS. The two faces of reactive oxygen species in cancer. Annu Rev Cancer Biol. 2017;1:79–98.
Arner ESJ. Targeting the selenoprotein thioredoxin reductase 1 for anticancer therapy. Adv Cancer Res. 2017;136:139–51.
Yee PP, Wei Y, Kim SY, Lu T, Chih SY, Lawson C, et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat Commun. 2020;11:5424.
Zhao Z, Zhang KN, Wang Q, Li G, Zeng F, Zhang Y, et al. Chinese Glioma Genome Atlas (CGGA): a comprehensive resource with functional genomic data from chinese glioma patients. Genom Proteom Bioinform. 2021;19:1–12.
Gusev Y, Bhuvaneshwar K, Song L, Zenklusen JC, Fine H, Madhavan S. The REMBRANDT study, a large collection of genomic data from brain cancer patients. Sci Data. 2018;5:180158.
Sutherland RM. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988;240:177–84.
Sakurai A, Nishimoto M, Himeno S, Imura N, Tsujimoto M, Kunimoto M, et al. Transcriptional regulation of thioredoxin reductase 1 expression by cadmium in vascular endothelial cells: role of NF-E2-related factor-2. J Cell Physiol. 2005;203:529–37.
Hintze KJ, Wald KA, Zeng H, Jeffery EH, Finley JW. Thioredoxin reductase in human hepatoma cells is transcriptionally regulated by sulforaphane and other electrophiles via an antioxidant response element. J Nutr. 2003;133:2721–7.
Olszewski K, Barsotti A, Feng XJ, Momcilovic M, Liu KG, Kim JI, et al. Inhibition of glucose transport synergizes with chemical or genetic disruption of mitochondrial metabolism and suppresses TCA cycle-deficient tumors. Cell Chem Biol. 2022;29:423–35.e10.
Gencheva R, Arner ESJ. Thioredoxin reductase inhibition for cancer therapy. Annu Rev Pharm Toxicol. 2022;62:177–96.
Stafford WC, Peng X, Olofsson MH, Zhang X, Luci DK, Lu L, et al. Irreversible inhibition of cytosolic thioredoxin reductase 1 as a mechanistic basis for anticancer therapy. Sci Transl Med. 2018;10:eaaf7444.
Ianevski A, Giri AK, Aittokallio T. SynergyFinder 3.0: an interactive analysis and consensus interpretation of multi-drug synergies across multiple samples. Nucleic Acids Res. 2022;50:W739–43.
Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta. 2013;1830:4117–29.
Joly JH, Delfarah A, Phung PS, Parrish S, Graham NA. A synthetic lethal drug combination mimics glucose deprivation-induced cancer cell death in the presence of glucose. J Biol Chem. 2020;295:1350–65.
Liu X, Olszewski K, Zhang Y, Lim EW, Shi J, Zhang X, et al. Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer. Nat Cell Biol. 2020;22:476–86.
Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62.
Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radic Biol Med. 2014;66:75–87.
Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61.
Galluzzi L, Vitale I, Warren S, Adjemian S, Agostinis P, Martinez AB, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer. 2020;8:e000337.
Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–38.
Rigobello MP, Folda A, Dani B, Menabo R, Scutari G, Bindoli A. Gold(I) complexes determine apoptosis with limited oxidative stress in Jurkat T cells. Eur J Pharm. 2008;582:26–34.
Cox AG, Brown KK, Arner ES, Hampton MB. The thioredoxin reductase inhibitor auranofin triggers apoptosis through a Bax/Bak-dependent process that involves peroxiredoxin 3 oxidation. Biochem Pharm. 2008;76:1097–109.
Park SJ, Kim IS. The role of p38 MAPK activation in auranofin-induced apoptosis of human promyelocytic leukaemia HL-60 cells. Br J Pharm. 2005;146:506–13.
Marzano C, Gandin V, Folda A, Scutari G, Bindoli A, Rigobello MP. Inhibition of thioredoxin reductase by auranofin induces apoptosis in cisplatin-resistant human ovarian cancer cells. Free Radic Biol Med. 2007;42:872–81.
Zhong Z, Zhang C, Ni S, Ma M, Zhang X, Sang W, et al. NFATc1-mediated expression of SLC7A11 drives sensitivity to TXNRD1 inhibitors in osteoclast precursors. Redox Biol. 2023;63:102711.
Barsé L, Düchting P, Lupilov N, Bandow JE, Krämer U, Leichert LI. Auranofin induces disulfide bond-mimicking S-Au-S bonds in protein thiol pairs. bioRxiv. 2024:2024.01.15.575694.
Walter DM, Venancio OS, Buza EL, Tobias JW, Deshpande C, Gudiel AA, et al. Systematic in vivo inactivation of chromatin-regulating enzymes identifies Setd2 as a potent tumor suppressor in lung adenocarcinoma. Cancer Res. 2017;77:1719–29.
Kraynov VS, Chamberlain C, Bokoch GM, Schwartz MA, Slabaugh S, Hahn KM. Localized Rac activation dynamics visualized in living cells. Science. 2000;290:333–7.
Acknowledgements
We would like to thank members of the Li laboratory for helpful discussions; Dr. Han Chen from the Transmission Electron Microscopy Core and the Microscopy Imaging Core (RRID: SCR_021200); Mr. Michael Grillo from the Microscopy Imaging Core (RRID: SCR_022526) (Leica SP8 Confocal: 1S10OD010756-01A1 CB); Dr. Wesley Raup–Konsavage from the Drug Discovery, Development and Delivery core; Mr. Nate Sheaffer, Ms. Jade Vogel, Ms. Jianhong Zhang and Mr. Joseph Bednarczyk from the Flow Cytometry Core (RRID: SCR_021134); Dr. Sirisha Pochareddy from the Genomics Sciences Core (RRID: SCR_021123); the Mass Spectrometry Core Facility (RRID: SCR_017831); Ms. Gretchen Snavely and Ms. Erin Mattern from the Comparative Medicine Histopathology Core; and Dr. Nataliya Smith, Ms. Kristin Shuler and Mr. John Graybeal from the Department of Neurosurgery’s Neuroscience Research Institute Biorepository for assistance with sample handling and IRB submissions. We acknowledge support from the National Institutes of Neurological Disorders and Stroke (R01 NS109147 and NS119547 to WL) and the Four Diamonds (to PSU).
Author information
Authors and Affiliations
Contributions
MT and WL conceived the project and designed experiments. MT performed most of the experiments under the assistance of KD, SYK, ZQ, YG, DS, GP, JS, and WL. MT and WL wrote an original manuscript. All authors provided intellectual input and edited the manuscript. WL supervised all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, M., Dirks, K., Kim, S.Y. et al. Inhibition of thioredoxin reductase 1 sensitizes glucose-starved glioblastoma cells to disulfidptosis. Cell Death Differ 32, 598–612 (2025). https://doi.org/10.1038/s41418-024-01440-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-024-01440-0
This article is cited by
-
Exploiting metabolic cell death for cancer therapy
Nature Reviews Cancer (2026)
-
Disulfidptosis in tumor progression
Cell Death Discovery (2025)
-
Redox-driven cell death by disulfidptosis and its therapeutic potential
Nature Reviews Molecular Cell Biology (2025)
-
Disulfidptosis meets antitumour immunity
Nature Cell Biology (2025)
-
Engineering overexpressing SYNGR1 inhibited the progression of GBM cells by suppressing the intracellular FGF1-mediated LDs accumulation and cytoskeleton remodeling
Journal of Neuro-Oncology (2025)