Abstract
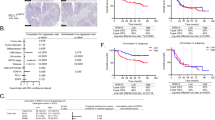
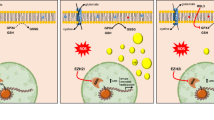
The mutation status of the lysine demethylase 6 A (KDM6A), a gene antagonist to Enhancer of zeste homolog 2 (EZH2), is closely related to the therapeutic efficacy of EZH2 inhibitors in several malignancies. However, the mutational landscape of KDM6A and the therapeutic targetability of EZH2 inhibitors in esophageal squamous carcinoma (ESCC) remain unreported. Here, we found that approximately 9.18% (9/98) of our study ESCC tissues had KDM6A mutations of which 7 cases resulted in a complete loss of expression and consequent loss of demethylase function. We found that KDM6A-deficient ESCC cells exhibited increased sensitivity to EZH2 inhibitor, and the radiosensitizing activity of EZH2 inhibitor was evident in KDM6A-dficient ESCC cells. Further transcriptome analysis revealed that ferroptosis is implicated in the radiosensitizing effect exerted by EZH2 inhibition on KDM6A-deficient ESCC cells. The following Chromatin Immunoprecipitation (ChIP), co-immunoprecipitation, and luciferase reporter assays demonstrated that in KDM6A-deficient ESCC cells, (1) Acyl-CoA Synthetase Long Chain Family Member 4 (ACSL4) is the target gene for EZH2 to regulate ferroptosis; (2) The IR-induced hypoxia inducible factor 1 subunit alpha (HIF-1α) is a predominant mediator of EZH2 to repress ACSL4; (3) the HRE7-8 regions of the ACSL4 promoter are required for the repressive function of EZH2 on ACSL4; (4) EZH2 regulates ACSL4 by forming a co-repressive complex with HIF-1α. Our study provides preclinical evidence supporting that EZH2 inhibitors may confer therapeutic benefit in KDM6A-deficient ESCC patients.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
RNA-Seq data generated in this study is publicly available in the Gene Expression Omnibus (GEO) at GSE264079. Derived data supporting the findings of this study are available from the corresponding author upon request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Wu H, Yan Z, Zhang J, Sun X, Long H, Li D, et al. Correlation of CT texture changes with treatment response during radiation therapy for esophageal cancer. Int J Rad Oncol Biol Phys. 2019;105:E202–E3.
Cohen DJ, Leichman L. Controversies in the treatment of local and locally advanced gastric and esophageal cancers. J Clin Oncol. 2015;33:1754–9.
Deng F, Zhou K, Cui W, Liu D, Ma Y. Clinicopathological significance of wnt/β-catenin signaling pathway in esophageal squamous cell carcinoma. Int J Clin Exper Pathol. 2015;8:3045.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–85.
Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–8.
Yuan Z-h, Liu T, Wang H, Xue L-x, Wang J-j. Fatty acids metabolism: the bridge between ferroptosis and ionizing radiation. Front Cell Dev Biol. 2021;9:675617.
Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12:599–620.
Ye LF, Chaudhary KR, Zandkarimi F, Harken AD, Kinslow CJ, Upadhyayula PS, et al. Radiation-induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers. ACS Chem Biol. 2020;15:469–84.
Kim EJ, Lee Y-J, Kang S, Lim Y-B. Ionizing radiation activates PERK/eIF2α/ATF4 signaling via ER stress-independent pathway in human vascular endothelial cells. Int J Radiat Biol. 2014;90:306–12.
Lang X, Green MD, Wang W, Yu J, Choi JE, Jiang L, et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 2019;9:1673–85.
Lei G, Zhang Y, Koppula P, Liu X, Zhang J, Lin SH, et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020;30:146–62.
Duan R, Du W, Guo W. EZH2: a novel target for cancer treatment. J Hematol Oncol. 2020;13:104.
Liu N, Konuma T, Sharma R, Wang D, Zhao N, Cao L, et al. Histone H3 lysine 27 crotonylation mediates gene transcriptional repression in chromatin. Mol Cell. 2023;83:2206–21.e11.
He LR, Liu MZ, Li BK, Jia WH, Zhang Y, Liao YJ, et al. High expression of EZH2 is associated with tumor aggressiveness and poor prognosis in patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Int J Cancer. 2010;127:138–47.
Huang X, Yan J, Zhang M, Wang Y, Chen Y, Fu X, et al. Targeting epigenetic crosstalk as a therapeutic strategy for EZH2-aberrant solid tumors. Cell. 2018;175:186–99.e19.
Li C, Wang Y, Gong Y, Zhang T, Huang J, Tan Z, et al. Finding an easy way to harmonize: a review of advances in clinical research and combination strategies of EZH2 inhibitors. Clin Epigenetics. 2021;13:62.
Pugh TJ, Weeraratne SD, Archer TC, Pomeranz Krummel DA, Auclair D, Bochicchio J, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488:106–10.
van Haaften G, Dalgliesh GL, Davies H, Chen L, Bignell G, Greenman C, et al. Somatic mutations of the histone H3K27 demethylase gene UTX in human cancer. Nat Genet. 2009;41:521–3.
Jankowska AM, Makishima H, Tiu RV, Szpurka H, Huang Y, Traina F, et al. Mutational spectrum analysis of chronic myelomonocytic leukemia includes genes associated with epigenetic regulation: UTX, EZH2, and DNMT3A. Blood. 2011;118:3932–41.
Ler LD, Ghosh S, Chai X, Thike AA, Heng HL, Siew EY, et al. Loss of tumor suppressor KDM6A amplifies PRC2-regulated transcriptional repression in bladder cancer and can be targeted through inhibition of EZH2. Sci Transl Med. 2017;9:984–5.
Ezponda T, Dupere-Richer D, Will CM, Small EC, Varghese N, Patel T, et al. UTX/KDM6A loss enhances the malignant phenotype of multiple myeloma and sensitizes cells to EZH2 inhibition. Cell Rep. 2017;21:628–40.
Gao YB, Chen ZL, Li JG, Hu XD, Shi XJ, Sun ZM, et al. Genetic landscape of esophageal squamous cell carcinoma. Nat Genet. 2014;46:1097–102.
Lin DC, Hao JJ, Nagata Y, Xu L, Shang L, Meng X, et al. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat Genet. 2014;46:467–73.
Cancer Genome Atlas Research N, Analysis Working Group: Asan U, Agency BCC, Brigham, Women’s H, Broad I. et al. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169–75.
Tong ZT, Cai MY, Wang XG, Kong LL, Mai SJ, Liu YH, et al. EZH2 supports nasopharyngeal carcinoma cell aggressiveness by forming a co-repressor complex with HDAC1/HDAC2 and Snail to inhibit E-cadherin. Oncogene. 2012;31:583–94.
Cai MY, Tong ZT, Zheng F, Liao YJ, Wang Y, Rao HL, et al. EZH2 protein: a promising immunomarker for the detection of hepatocellular carcinomas in liver needle biopsies. Gut. 2011;60:967–76.
Tong Z, Fang W, Xu M, Xia Y, Wang R, Li Y, et al. DAB2IP predicts treatment response and prognosis of ESCC patients and modulates its radiosensitivity through enhancing IR-induced activation of the ASK1-JNK pathway. Cancer Cell Int. 2022;22:106.
Sak A, Stuschke M. Use of gammaH2AX and other biomarkers of double-strand breaks during radiotherapy. Semin Radiat Oncol. 2010;20:223–31.
Schultz LB, Chehab NH, Malikzay A, Halazonetis TD. p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J Cell Biol. 2000;151:1381–90.
Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–7.
Meyn RE, Milas L, Ang KK. The role of apoptosis in radiation oncology. Int J Radiat Biol. 2009;85:107–15.
Muller J, Kassis JA. Polycomb response elements and targeting of Polycomb group proteins in Drosophila. Curr Opin Genet Dev. 2006;16:476–84.
Wilkinson F, Pratt H, Atchison ML. PcG recruitment by the YY1 REPO domain can be mediated by Yaf2. J Cellular Biochem. 2010;109:478–86.
Jiang Y Fasting alters histone methylation in paraventricular nucleus of chick through regulating of polycomb repressive complex 2: Virginia Tech; 2013.
Quan J, Bode AM, Luo X. ACSL family: The regulatory mechanisms and therapeutic implications in cancer. Eur J Pharmacol. 2021;909:174397.
Moeller BJ, Dewhirst MW. HIF-1 and tumour radiosensitivity. Br J Cancer. 2006;95:1–5.
Masoud GN, Li W. HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378–89.
Li Y, Zhao X, Tang H, Zhong Z, Zhang L, Xu R, et al. Effects of YC-1 on hypoxia-inducible factor 1 alpha in hypoxic human bladder transitional carcinoma cell line T24 cells. Urol Int. 2012;88:95–101.
Ouyang C, Zhang J, Lei X, Xie Z, Liu X, Li Y, et al. Advances in antitumor research of HIF-1α inhibitor YC-1 and its derivatives. Bioog Chem. 2023;133:106400.
Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378–89.
Wu Q, You L, Nepovimova E, Heger Z, Wu W, Kuca K, et al. Hypoxia-inducible factors: master regulators of hypoxic tumor immune escape. J Hematol Oncol. 2022;15:77.
Liu GZ, Xu XW, Tao SH, Gao MJ, Hou ZH. HBx facilitates ferroptosis in acute liver failure via EZH2 mediated SLC7A11 suppression. J Biomed Sci. 2021;28:67.
Yi C, Li G, Wang W, Sun Y, Zhang Y, Zhong C, et al. Disruption of YY1-EZH2 interaction using synthetic peptides inhibits breast cancer development. Cancers. 2021;13:2402.
Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–41.
Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–13.
Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–53.
Kim SH, Joshi K, Ezhilarasan R, Myers TR, Siu J, Gu C, et al. EZH2 protects glioma stem cells from radiation-induced cell death in a MELK/FOXM1-dependent manner. Stem Cell Rep. 2015;4:226–38.
Zhang X, Ma X, Wang Q, Kong Z. EZH2 targeting to improve the sensitivity of acquired radio-resistance bladder cancer cells. Transl Oncol. 2022;16:101316.
Acknowledgements
We are grateful to Dr. Jiabing Fan (University of California, Los Angeles) for his invaluable comments and extensive manuscript editing. We also thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
This study was supported by the University Natural Science Research Project of Anhui Province (Grant Number: KJ2021A0295), the National Natural Science Foundation of China (Grant Number: 81201743), the Health Research Program of Anhui (Grant Number: AHWJ2023BAc20040), and the Natural Science Foundation of Anhui Province, China (Grant Number: 1908085QA27).
Author information
Authors and Affiliations
Contributions
ZT conceived and designed the study. GP, YX, and MH performed most of the experiments. WF, JG, LS, SZ, and TZ assisted in the performance of the experiments. HP, XX, QZ, and XC collected patient samples. ZT, GP, YX, MH, and JG contributed to data analysis. ZT, GP, and YX prepared and revised the figures, and drafted the manuscript with assistance from JG and MH. ZT, XC, and QZ provided funding. ZT supervised the research. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
Approval for human tissue samples utilization was granted by the Biomedical Ethics Committee of Anhui Medical University (5101745), following the guidelines of Declaration of Helsinki. All patients authorized the use of their specimens by written informed consent. All animal experiments were carried out with the approval of the Experimental Animal Ethical Committee of Anhui Medical University (LLSC20210863) and performed according to institutional guidelines.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, G., Xia, Y., Hao, M. et al. EZH2 suppresses IR-induced ferroptosis by forming a co-repressor complex with HIF-1α to inhibit ACSL4: Targeting EZH2 enhances radiosensitivity in KDM6A-deficient esophageal squamous carcinoma. Cell Death Differ 32, 1026–1040 (2025). https://doi.org/10.1038/s41418-025-01451-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-025-01451-5
This article is cited by
-
Ubiquitin choreography in nasopharyngeal carcinoma: USP18 scaffolds radioresistance
Cell Death & Differentiation (2025)
-
Immune cells dying from ferroptosis: mechanisms and therapeutic opportunities
Cell Death & Disease (2025)