Abstract
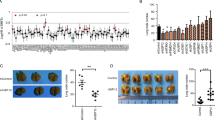
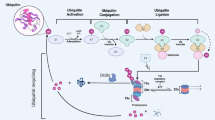
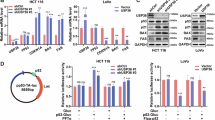
Dysregulation of the MDM2-p53 pathway is a commonly observed phenomenon in cancer, where overexpression or amplification of MDM2 leads to increased degradation of p53. This results in reduced levels of p53, leading to the loss of its tumor-suppressive functions. The study focused on investigating the role of Ubiquitin-specific protease 38 (USP38) in cancer and its interaction with the MDM2-p53 axis. We revealed that USP38 positively correlates with MDM2 and negatively correlates with p53 expression. Mechanistically, USP38 directly binds to MDM2, functioning as a deubiquitinating enzyme (DUB) to stabilize MDM2 and suppress p53 expression. Knockout of USP38 hindered cancer cell proliferation, migration, and invasion, and enhanced apoptosis. Moreover, USP38 deficiency increased sensitivity to chemotherapy drugs and promoted ferroptosis in gastric and breast cancer cell lines. Importantly, these effects were found to be dependent on p53, as the downregulation of p53 reversed the phenotypic changes induced by USP38 knockout. These findings shed light on the oncogenic role of USP38 by modulating the MDM2-p53 axis, providing valuable insights into the molecular mechanisms of USP38 in cancer and potential therapeutic strategies for gastric and breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Information on experimental reagents and primer sequences can be found in the Supplementary Tables. All other data are available from the corresponding authors upon request.
References
Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303.
Michael D, Oren M. The p53-Mdm2 module and the ubiquitin system. Semin Cancer Biol. 2003;13:49–58.
Marine JC, Lozano G. Mdm2-mediated ubiquitylation: p53 and beyond. Cell Death Differ. 2010;17:93–102.
Brooks CL, Gu W. p53 ubiquitination: Mdm2 and beyond. Mol Cell. 2006;21:307–15.
Li M, Chen D, Shiloh A, Luo J, Nikolaev AY, Qin J, et al. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature. 2002;416:648–53.
Li M, Brooks CL, Kon N, Gu W. A dynamic role of HAUSP in the p53-Mdm2 pathway. Mol Cell. 2004;13:879–86.
Stevenson LF, Sparks A, Allende-Vega N, Xirodimas DP, Lane DP, Saville MK. The deubiquitinating enzyme USP2a regulates the p53 pathway by targeting Mdm2. Embo j. 2007;26:976–86.
Zou Q, Jin J, Hu H, Li HS, Romano S, Xiao Y, et al. USP15 stabilizes MDM2 to mediate cancer-cell survival and inhibit antitumor T cell responses. Nat Immunol. 2014;15:562–70.
Kwon SK, Saindane M, Baek KH. p53 stability is regulated by diverse deubiquitinating enzymes. Biochim Biophys Acta Rev Cancer. 2017;1868:404–11.
Tavana O, Gu W. Modulation of the p53/MDM2 interplay by HAUSP inhibitors. J Mol Cell Biol. 2017;9:45–52.
Chen L, Liu S, Tao Y. Regulating tumor suppressor genes: post-translational modifications. Signal Transduct Target Ther. 2020;5:90.
Wade M, Li YC, Wahl GM. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer. 2013;13:83–96.
Lin M, Zhao Z, Yang Z, Meng Q, Tan P, Xie W, et al. USP38 Inhibits Type I Interferon Signaling by Editing TBK1 Ubiquitination through NLRP4 Signalosome. Mol Cell. 2016;64:267–81.
Wang H, Xue X. USP38 protein alleviates neuroinflammation of cerebral ischemia–reperfusion injury via KDM5B expression. Molecular Cellular Toxicology. 2021;17:465–73.
Zhao Z, Su Z, Liang P, Liu D, Yang S, Wu Y, et al. USP38 Couples Histone Ubiquitination and Methylation via KDM5B to Resolve Inflammation. Adv Sci (Weinh). 2020;7:2002680.
Wang R, Cai X, Li X, Li J, Liu X, Wang J, et al. USP38 promotes deubiquitination of K11-linked polyubiquitination of HIF1α at Lys769 to enhance hypoxia signaling. J Biol Chem. 2024;300:105532.
Zhan W, Liao X, Liu J, Tian T, Yu L, Li R. USP38 regulates the stemness and chemoresistance of human colorectal cancer via regulation of HDAC3. Oncogenesis. 2020;9:48.
Xu Z, Hu H, Fang D, Wang J, Zhao K. The deubiquitinase USP38 promotes cell proliferation through stabilizing c-Myc. Int J Biochem Cell Biol. 2021;137:106023.
Wang Y, Li Q, Hu D, Gao D, Wang W, Wu K, et al. USP38 Inhibits Zika Virus Infection by Removing Envelope Protein Ubiquitination. Viruses. 2021;13:2029.
Yang Y, Yang C, Li T, Yu S, Gan T, Hu J, et al. The Deubiquitinase USP38 Promotes NHEJ Repair through Regulation of HDAC1 Activity and Regulates Cancer Cell Response to Genotoxic Insults. Cancer Res. 2020;80:719–31.
Zhang T, Su F, Wang B, Liu L, Lu Y, Su H, et al. Ubiquitin specific peptidase 38 epigenetically regulates KLF transcription factor 5 to augment malignant progression of lung adenocarcinoma. Oncogene. 2024;43:1190–202.
Ru B, Wong CN, Tong Y, Zhong JY, Zhong SSW, Wu WC, et al. TISIDB: an integrated repository portal for tumor-immune system interactions. Bioinformatics. 2019;35:4200–2.
Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47:W556–60.
Pratt EP, Owens JL, Hockerman GH, Hu CD. Bimolecular Fluorescence Complementation (BiFC) Analysis of Protein-Protein Interactions and Assessment of Subcellular Localization in Live Cells. Methods Mol Biol. 2016;1474:153–70.
Ding L, Zhang Z, Zhao C, Chen L, Chen Z, Zhang J, et al. Ribosomal L1 domain-containing protein 1 coordinates with HDM2 to negatively regulate p53 in human colorectal Cancer cells. J Exp Clin Cancer Res. 2021;40:245.
Doherty LM, Mills CE, Boswell SA, Liu X, Hoyt CT, Gyori B, et al. Integrating multi-omics data reveals function and therapeutic potential of deubiquitinating enzymes. Elife. 2022;11:e72879.
Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62.
Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–8.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.
Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–25.
Liu W, Zhang Q, Fang Y, Wang Y. The deubiquitinase USP38 affects cellular functions through interacting with LSD1. Biol Res. 2018;51:53.
Zheng Z, Shang Y, Xu R, Yan X, Wang X, Cai J, et al. Ubiquitin specific peptidase 38 promotes the progression of gastric cancer through upregulation of fatty acid synthase. Am J Cancer Res. 2022;12:2686–96.
Liu J, Zhang C, Wang J, Hu W, Feng Z. The Regulation of Ferroptosis by Tumor Suppressor p53 and its Pathway. Int J Mol Sci. 2020;21:8387.
Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17:2054–81.
Funding
This work was in part supported by the National Natural Science Foundation of China (No. 81872260, 82172938 to PZZ), the Shanghai Natural Science Foundation (No. 21ZR1450300 to XPL), the National Key Clinical Specialties-Pathology (No. YWP2023-001 to YYH).
Author information
Authors and Affiliations
Contributions
PZZ and XPL conceived the study. SYZ, XLL, RKL, ZTJ, CX performed the experiments and data analyses. PZZ, XPL, YYH analyzed and interpreted the data. PZZ and SYZ wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
This study involved human subjects and animal experiments and was approved by the Ethics Review Committee of Fudan University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, S., Liu, X., Luo, R. et al. USP38 functions as an oncoprotein by downregulating the p53 pathway through deubiquitination and stabilization of MDM2. Cell Death Differ 32, 1128–1141 (2025). https://doi.org/10.1038/s41418-025-01462-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-025-01462-2