Abstract
The protein branched-chain ketoacid dehydrogenase kinase (BCKDK), which regulates the metabolism of branched-chain amino acids, has recently been implicated in tumor progression. However, the role of BCKDK in lung cancer remains largely unexplored. In this study, we explored the mechanisms by which BCKDK influences lung cancer progression and contributes to drug resistance. By integrating single-cell RNA and bulk RNA sequencing data from lung cancer patients, we identified BCKDK as a novel gene related to malignant epithelial cells, involved in tumor initiation and associated with poor patient prognosis. Subsequently, through a series of molecular biology experiments, we demonstrated that BCKDK promotes aerobic glycolysis, Trametinib resistance, and tumor progression in lung cancer by upregulating MYC transcription. Mechanistically, BCKDK interacts with BCLAF1 to promote its phosphorylation at the serine 285 site. This modification facilitates BCLAF1 binding to the MYC promoter, thereby enhancing MYC transcription. Subsequently, elevated MYC levels upregulate hexokinase 2, promoting aerobic glycolysis and lung cancer progression. In addition, the elevated glycolysis product, lactate, promotes Trametinib resistance by upregulating the ABC transporters. Taken together, our data identify BCKDK as a novel regulator of aerobic glycolysis that promotes lung cancer progression and Trametinib resistance through the BCKDK/BCLAF1/MYC/HK2 axis. Targeting BCKDK in combination with Trametinib may offer a promising treatment for lung cancer.

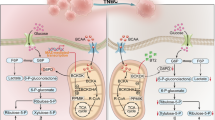
Graphical representation of the BCKDK/BCLAF1/MYC/HK2 axis and its role in Trametinib resistance and lung cancer progression. Created with BioRender.com.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63.
Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–54.
Spicer JD, Cascone T, Wynes MW, Ahn MJ, Dacic S, Felip E, et al. Neoadjuvant and Adjuvant Treatments for Early Stage Resectable NSCLC: Consensus Recommendations From the International Association for the Study of Lung Cancer. J Thorac Oncol. 2024;19:1373–414.
Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. 2021;27:1345–56.
Harris RA, Hawes JW, Popov KM, Zhao Y, Shimomura Y, Sato J, et al. Studies on the regulation of the mitochondrial alpha-ketoacid dehydrogenase complexes and their kinases. Adv Enzym Regul. 1997;37:271–93.
Zhai M, Yang Z, Zhang C, Li J, Jia J, Zhou L, et al. APN-mediated phosphorylation of BCKDK promotes hepatocellular carcinoma metastasis and proliferation via the ERK signaling pathway. Cell Death Dis. 2020;11:396.
Ericksen RE, Lim SL, McDonnell E, Shuen WH, Vadiveloo M, White PJ, et al. Loss of BCAA Catabolism during Carcinogenesis Enhances mTORC1 Activity and Promotes Tumor Development and Progression. Cell Metab. 2019;29:1151–65.e6.
Xue M, Xiao J, Jiang W, Wang Y, Zuo D, An H, et al. Loss of BCAA catabolism enhances Rab1A-mTORC1 signaling activity and promotes tumor proliferation in NSCLC. Transl Oncol. 2023;34:101696.
Xue P, Zeng F, Duan Q, Xiao J, Liu L, Yuan P, et al. BCKDK of BCAA Catabolism Cross-talking With the MAPK Pathway Promotes Tumorigenesis of Colorectal Cancer. EBioMedicine. 2017;20:50–60.
Li H, Yu D, Li L, Xiao J, Zhu Y, Liu Y, et al. BCKDK Promotes Ovarian Cancer Proliferation and Migration by Activating the MEK/ERK Signaling Pathway. J Oncol. 2022;2022:3691635.
Wang Y, Xiao J, Jiang W, Zuo D, Wang X, Jin Y, et al. BCKDK alters the metabolism of non-small cell lung cancer. Transl Lung Cancer Res. 2021;10:4459–76.
Chen H, Li Y, Li H, Chen X, Fu H, Mao D, et al. NBS1 lactylation is required for efficient DNA repair and chemotherapy resistance. Nature. 2024;631:663–9.
Sun X, He L, Liu H, Thorne RF, Zeng T, Liu L, et al. The diapause-like colorectal cancer cells induced by SMC4 attenuation are characterized by low proliferation and chemotherapy insensitivity. Cell Metab. 2023;35:1563–79.e8.
Mathupala SP, Ko YH, Pedersen PL. Hexokinase-2 bound to mitochondria: cancer’s stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin Cancer Biol. 2009;19:17–24.
Kim JW, Gao P, Liu YC, Semenza GL, Dang CV. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol. 2007;27:7381–93.
Kumar R, Chaudhary AK, Woytash J, Inigo JR, Gokhale AA, Bshara W, et al. A mitochondrial unfolded protein response inhibitor suppresses prostate cancer growth in mice via HSP60. J Clin Invest. 2022;132:e149906.
Zhou X, Wen Y, Tian Y, He M, Ke X, Huang Z, et al. Heat Shock Protein 90α-Dependent B-Cell-2-Associated Transcription Factor 1 Promotes Hepatocellular Carcinoma Proliferation by Regulating MYC Proto-Oncogene c-MYC mRNA Stability. Hepatology. 2019;69:1564–81.
Tan W, Zhang J, Liu L, Liang M, Li J, Deng Z, et al. Hsp90 Inhibitor STA9090 induced VPS35 related extracellular vesicle release and metastasis in hepatocellular carcinoma. Transl Oncol. 2022;26:101502.
Wen Y, Zhou X, Lu M, He M, Tian Y, Liu L, et al. Bclaf1 promotes angiogenesis by regulating HIF-1α transcription in hepatocellular carcinoma. Oncogene. 2019;38:1845–59.
Kasof GM, Goyal L, White E. Btf, a novel death-promoting transcriptional repressor that interacts with Bcl-2-related proteins. Mol Cell Biol. 1999;19:4390–404.
Shao AW, Sun H, Geng Y, Peng Q, Wang P, Chen J, et al. Bclaf1 is an important NF-κB signaling transducer and C/EBPβ regulator in DNA damage-induced senescence. Cell Death Differ. 2016;23:865–75.
Wang J, Zhu X, Wang S, Zhang Y, Hua W, Liu Z, et al. Phosphoproteomic and proteomic profiling in post-infarction chronic heart failure. Front Pharm. 2023;14:1181622.
Liu J, Li J, Sun Z, Duan Y, Wang F, Wei G, et al. Bcl-2-associated transcription factor 1 Ser290 phosphorylation mediates DNA damage response and regulates radiosensitivity in gastric cancer. J Transl Med. 2021;19:339.
Lee YY, Yu YB, Gunawardena HP, Xie L, Chen X. BCLAF1 is a radiation-induced H2AX-interacting partner involved in γH2AX-mediated regulation of apoptosis and DNA repair. Cell Death Dis. 2012;3:e359.
Chen J, Guanizo AC, Jakasekara WSN, Inampudi C, Luong Q, Garama DJ, et al. MYC drives platinum resistant SCLC that is overcome by the dual PI3K-HDAC inhibitor fimepinostat. J Exp Clin Cancer Res. 2023;42:100.
Macaya I, Roman M, Welch C, Entrialgo-Cadierno R, Salmon M, Santos A, et al. Signature-driven repurposing of Midostaurin for combination with MEK1/2 and KRASG12C inhibitors in lung cancer. Nat Commun. 2023;14:6332.
Zhang Q, Shi Y, Liu S, Yang W, Chen H, Guo N, et al. EZH2/G9a interact to mediate drug resistance in non-small-cell lung cancer by regulating the SMAD4/ERK/c-Myc signaling axis. Cell Rep. 2024;43:113714.
Wang S, Zhou L, Ji N, Sun C, Sun L, Sun J, et al. Targeting ACYP1-mediated glycolysis reverses lenvatinib resistance and restricts hepatocellular carcinoma progression. Drug Resist Updat. 2023;69:100976.
Li X, Zhang Y, Wang X, Lin F, Cheng X, Wang Z, et al. Long non-coding RNA CTSLP8 mediates ovarian cancer progression and chemotherapy resistance by modulating cellular glycolysis and regulating c-Myc expression through PKM2. Cell Biol Toxicol. 2022;38:1027–45.
Jiang X, Guo S, Wang S, Zhang Y, Chen H, Wang Y, et al. EIF4A3-Induced circARHGAP29 Promotes Aerobic Glycolysis in Docetaxel-Resistant Prostate Cancer through IGF2BP2/c-Myc/LDHA Signaling. Cancer Res. 2022;82:831–45.
Lee HJ, Li CF, Ruan D, He J, Montal ED, Lorenz S, et al. Non-proteolytic ubiquitination of Hexokinase 2 by HectH9 controls tumor metabolism and cancer stem cell expansion. Nat Commun. 2019;10:2625.
Odogwu L, Mathieu L, Blumenthal G, Larkins E, Goldberg KB, Griffin N, et al. FDA Approval summary: dabrafenib and trametinib for the treatment of metastatic non-small cell lung cancers harboring BRAF V600E mutations. Oncologist. 2018;23:740–5.
Hanrahan AJ, Chen Z, Rosen N, Solit DB. BRAF - a tumour-agnostic drug target with lineage-specific dependencies. Nat Rev Clin Oncol. 2024;21:224–47.
Marampon F, Ciccarelli C, Zani BM. Down-regulation of c-Myc following MEK/ERK inhibition halts the expression of malignant phenotype in rhabdomyosarcoma and in non muscle-derived human tumors. Mol Cancer. 2006;5:31.
Kerkhoff E, Houben R, Löffler S, Troppmair J, Lee JE, Rapp UR. Regulation of c-myc expression by Ras/Raf signalling. Oncogene. 1998;16:211–6.
Xu C, Yang K, Xuan Z, Li J, Liu Y, Zhao Y, et al. BCKDK regulates breast cancer cell adhesion and tumor metastasis by inhibiting TRIM21 ubiquitinate talin1. Cell Death Dis. 2023;14:445.
Silvis MR, Silva D, Rohweder R, Schuman S, Gudipaty S, Truong A, et al. MYC-mediated resistance to trametinib and HCQ in PDAC is overcome by CDK4/6 and lysosomal inhibition. J Exp Med. 2023;220:e20221524.
Ravichandran M, Hu J, Cai C, Ward NP, Venida A, Foakes C, et al. Coordinated Transcriptional and catabolic programs support iron-dependent adaptation to RAS-MAPK pathway inhibition in pancreatic cancer. Cancer Discov. 2022;12:2198–219.
Gao M, Yang J, Gong H, Lin Y, Liu J. Trametinib Inhibits the Growth and Aerobic Glycolysis of Glioma Cells by Targeting the PKM2/c-Myc Axis. Front Pharm. 2021;12:760055.
Lebedev TD, Khabusheva ER, Mareeva SR, Ivanenko KA, Morozov AV, Spirin PV, et al. Identification of cell type-specific correlations between ERK activity and cell viability upon treatment with ERK1/2 inhibitors. J Biol Chem. 2022;298:102226.
Ghandi M, Huang FW, Jané-Valbuena J, Kryukov GV, Lo CC, McDonald ER, et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature. 2019;569:503–8.
Murphy BM, Terrell EM, Chirasani VR, Weiss TJ, Lew RE, Holderbaum AM, et al. Enhanced BRAF engagement by NRAS mutants capable of promoting melanoma initiation. Nat Commun. 2022;13:3153.
Han L, Czech J, Maurer A, Brümmendorf TH, Chatain N, Koschmieder S. Mutant NRAS Q61K is responsible for MAPK pathway activation in the MARIMO cell line and renders these cells independent of the CALR-MPL-JAK2-STAT5 pathway. Leukemia. 2018;32:2087–90.
Abravanel DL, Nishino M, Sholl LM, Ambrogio C, Awad MM. An Acquired NRAS Q61K Mutation in BRAF V600E-Mutant Lung Adenocarcinoma Resistant to Dabrafenib Plus Trametinib. J Thorac Oncol. 2018;13:e131–e3.
Coelho D, Kim JC, Miousse IR, Fung S, du Moulin M, Buers I, et al. Mutations in ABCD4 cause a new inborn error of vitamin B12 metabolism. Nat Genet. 2012;44:1152–5.
Napier I, Ponka P, Richardson DR. Iron trafficking in the mitochondrion: novel pathways revealed by disease. Blood. 2005;105:1867–74.
Po A, Citarella A, Catanzaro G, Besharat ZM, Trocchianesi S, Gianno F, et al. Hedgehog-GLI signalling promotes chemoresistance through the regulation of ABC transporters in colorectal cancer cells. Sci Rep. 2020;10:13988.
Panneerselvam J, Mohandoss P, Patel R, Gillan H, Li M, Kumar K, et al. DCLK1 Regulates Tumor Stemness and Cisplatin Resistance in Non-small Cell Lung Cancer via ABCD-Member-4. Mol Ther Oncolytics. 2020;18:24–36.
Acknowledgements
We would like to thank the research sharing platform of Tianjin Medical University for providing experimental assistance.
Funding
This work was funded by the National Key Technologies R&D Program (intergovernmental international innovation cooperation, 2018YFE0102000) and the National Natural Science Foundation of China (NSFC) Program (82173199).
Author information
Authors and Affiliations
Contributions
HW performed and analyzed all bioinformatic analyses and experiments. JJY participated in most of the experiments. ZXY was responsible for tissue microarray acquisition and immunohistochemistry experiments. YWX contributed to data acquisition and analysis. RL, JJ, XRZ and YTZ supported some of the experiments. HW wrote the manuscript. ZY and ZF provided critical resources and financial support. JQL conceptualized the project, supervised the study, and secured funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
Human tumor tissues were sourced from Tianjin Medical University General Hospital. Informed consent was obtained from all patients before participation. The study involving human subjects was approved by the Ethics Committee of Tianjin Medical University General Hospital and conducted in compliance with the Declaration of Helsinki. Additionally, all animal experiments were reviewed and approved by the Animal Ethics Committee of Tianjin Medical University (TMUaMEC2024057) and performed in accordance with its legal requirements.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, H., Yang, J., Yang, Z. et al. Targeting the BCKDK/BCLAF1/MYC/HK2 axis to alter aerobic glycolysis and overcome Trametinib resistance in lung cancer. Cell Death Differ 32, 2210–2224 (2025). https://doi.org/10.1038/s41418-025-01531-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-025-01531-6