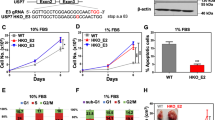
Abstract
M2-like tumor-associated macrophages (TAMs) are the main immunosuppressive cells infiltrating the tumor microenvironment (TME), the activation of which is essential for cancer progression and resistance promotion to immunotherapy. However, the regulatory mechanisms underlying TAM activation have not been fully elucidated. Utilizing a CRISPR-Cas9-based genetically engineered mouse model, we discovered that USP1fl/flLyz2cre/+ and WDR48fl/flLyz2cre/+ mice exhibited decreased tumor formation and lung metastasis. Mechanistically, the USP1-WDR48 deubiquitinase complex regulated M2-TAM activation and infiltration in the TME by modulating DDX3X ubiquitination. Specifically, this complex interacted with the N-terminal RecA-like domain 1 of DDX3X, leading to K48-linked deubiquitination and stabilization of DDX3X. Then, DDX3X promoted the translation of signaling molecules Jak1 and Rac1 via its RNA helicase activity, activating the Jak1-Stat3/6 and Rac1-Akt pathways to drive M2-TAM activation. Furthermore, combined inhibition of the USP1/WDR48 and CD47/SIRPα signaling pathways showed synergistic antitumor effects in immunocompetent mice. Notably, USP1 protein expression in tumor stromal tissues independently predicts prognosis in breast cancer patients. These findings indicated the role of the USP1-WDR48 complex as a critical molecular switch controlling TAM activation, presenting novel and promising targets for breast cancer treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The LC–MS/MS data and uncropped images of immunoblots are included in the supplement. If necessary, more data can be provided from the corresponding author upon reasonable request.
References
Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51.
Cassetta L, Pollard JW. Tumor-associated macrophages. Curr Biol. 2020;30:R246–r8.
Zhang X, Li S, Malik I, Do MH, Ji L, Chou C, et al. Reprogramming tumour-associated macrophages to outcompete cancer cells. Nature. 2023;619:616–23.
Wang Q, Bergholz JS, Ding L, Lin Z, Kabraji SK, Hughes ME, et al. STING agonism reprograms tumor-associated macrophages and overcomes resistance to PARP inhibition in BRCA1-deficient models of breast cancer. Nat Commun. 2022;13:3022.
Zhang W, Zhang Q, Yang N, Shi Q, Su H, Lin T, et al. Crosstalk between IL-15Rα(+) tumor-associated macrophages and breast cancer cells reduces CD8(+) T cell recruitment. Cancer Commun. 2022;42:536–57.
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416.
Hourani T, Sharma A, Luwor RB, Achuthan AA. Transforming growth factor-β in tumor microenvironment: understanding its impact on monocytes and macrophages for its targeting. Int Rev Immunol. 2025;44:82–97.
Zhou J, Lyu N, Wang Q, Yang M, Kimchi ET, Cheng K, et al. A novel role of TGFBI in macrophage polarization and macrophage-induced pancreatic cancer growth and therapeutic resistance. Cancer Lett. 2023;578:216457.
Sun H, Miao C, Liu W, Qiao X, Yang W, Li L, et al. TGF-β1/TβRII/Smad3 signaling pathway promotes VEGF expression in oral squamous cell carcinoma tumor-associated macrophages. Biochem Biophys Res Commun. 2018;497:583–90.
Shen Z, Seppänen H, Kauttu T, Vainionpää S, Ye Y, Wang S, et al. Vasohibin-1 expression is regulated by transforming growth factor-β/bone morphogenic protein signaling pathway between tumor-associated macrophages and pancreatic cancer cells. J Interferon Cytokine Res. 2013;33:428–33.
Arwert EN, Harney AS, Entenberg D, Wang Y, Sahai E, Pollard JW, et al. A unidirectional transition from migratory to perivascular macrophage is required for tumor cell intravasation. Cell Rep. 2018;23:1239–48.
Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604.
Fu JL, Hao HF, Wang S, Jiao YN, Li PP, Han SY. Marsdenia tenacissima extract disturbs the interaction between tumor-associated macrophages and non-small cell lung cancer cells by targeting HDGF. J Ethnopharmacol. 2022;298:115607.
Wang C, Ma C, Gong L, Guo Y, Fu K, Zhang Y, et al. Macrophage polarization and its role in liver disease. Front Immunol. 2021;12:803037.
He L, Jhong JH, Chen Q, Huang KY, Strittmatter K, Kreuzer J, et al. Global characterization of macrophage polarization mechanisms and identification of M2-type polarization inhibitors. Cell Rep. 2021;37:109955.
Chen S, Wang M, Lu T, Liu Y, Hong W, He X, et al. JMJD6 in tumor-associated macrophage regulates macrophage polarization and cancer progression via STAT3/IL-10 axis. Oncogene. 2023;42:2737–50.
Niu F, Yu Y, Li Z, Ren Y, Li Z, Ye Q, et al. Arginase: an emerging and promising therapeutic target for cancer treatment. Biomed Pharmacother. 2022;149:112840.
Massagué J. TGFbeta in cancer. Cell. 2008;134:215–30.
Novitskiy SV, Pickup MW, Gorska AE, Owens P, Chytil A, Aakre M, et al. TGF-β receptor II loss promotes mammary carcinoma progression by Th17 dependent mechanisms. Cancer Discov. 2011;1:430–41.
Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharm. 2020;877:173090.
Lundahl MLE, Mitermite M, Ryan DG, Case S, Williams NC, Yang M, et al. Macrophage innate training induced by IL-4 and IL-13 activation enhances OXPHOS driven anti-mycobacterial responses. eLife. 2022;11:e74690.
Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–103.
Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, Sieling PA, et al. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291:1544–7.
Wu KK, Xu X, Wu M, Li X, Hoque M, Li GHY, et al. MDM2 induces pro-inflammatory and glycolytic responses in M1 macrophages by integrating iNOS-nitric oxide and HIF-1α pathways in mice. Nat Commun. 2024;15:8624.
Logtenberg MEW, Scheeren FA, Schumacher TN. The CD47-SIRPα immune checkpoint. Immunity. 2020;52:742–52.
van Duijn A, Van der Burg SH, Scheeren FA. CD47/SIRPα axis: bridging innate and adaptive immunity. J Immunother Cancer. 2022;10:e004589.
Veillette A, Chen J. SIRPα-CD47 immune checkpoint blockade in anticancer therapy. Trends Immunol. 2018;39:173–84.
Weiskopf K. Cancer immunotherapy targeting the CD47/SIRPα axis. Eur J Cancer. 2017;76:100–9.
Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N Engl J Med. 2018;379:1711–21.
Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713.
Ring NG, Herndler-Brandstetter D, Weiskopf K, Shan L, Volkmer JP, George BM, et al. Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci USA. 2017;114:E10578–e85.
Liu Y, Weng L, Wang Y, Zhang J, Wu Q, Zhao P, et al. Deciphering the role of CD47 in cancer immunotherapy. J Adv Res. 2024;63:129–58.
Williams SA, Maecker HL, French DM, Liu J, Gregg A, Silverstein LB, et al. USP1 deubiquitinates ID proteins to preserve a mesenchymal stem cell program in osteosarcoma. Cell. 2011;146:918–30.
García-Santisteban I, Peters GJ, Giovannetti E, Rodríguez JA. USP1 deubiquitinase: cellular functions, regulatory mechanisms and emerging potential as target in cancer therapy. Mol Cancer. 2013;12:91.
Zhang DY, Zhu Y, Wu Q, Ma S, Ma Y, Shen ZC, et al. USP1 promotes cholangiocarcinoma progression by deubiquitinating PARP1 to prevent its proteasomal degradation. Cell Death Dis. 2023;14:669.
Liu D, Li Q, Zang Y, Li X, Li Z, Zhang P, et al. USP1 modulates hepatocellular carcinoma progression via the Hippo/TAZ axis. Cell Death Dis. 2023;14:264.
Li XY, Wu JC, Liu P, Li ZJ, Wang Y, Chen BY, et al. Inhibition of USP1 reverses the chemotherapy resistance through destabilization of MAX in the relapsed/refractory B-cell lymphoma. Leukemia. 2023;37:164–77.
Mallard HJ, Wan S, Nidhi P, Hanscom-Trofy YD, Mohapatra B, Woods NT, et al. USP1 expression driven by EWS::FLI1 transcription factor stabilizes survivin and mitigates replication stress in Ewing sarcoma. Mol Cancer Res. 2023;21:1186–204.
Liu S, Xiang Y, Wang B, Gao C, Chen Z, Xie S, et al. USP1 promotes the aerobic glycolysis and progression of T-cell acute lymphoblastic leukemia via PLK1/LDHA axis. Blood Adv. 2023;7:3099–112.
Sowa ME, Bennett EJ, Gygi SP, Harper JW. Defining the human deubiquitinating enzyme interaction landscape. Cell. 2009;138:389–403.
Cohn MA, Kee Y, Haas W, Gygi SP, D’Andrea AD. UAF1 is a subunit of multiple deubiquitinating enzyme complexes. J Biol Chem. 2009;284:5343–51.
Yu Z, Song H, Jia M, Zhang J, Wang W, Li Q, et al. USP1-UAF1 deubiquitinase complex stabilizes TBK1 and enhances antiviral responses. J Exp Med. 2017;214:3553–63.
Ge X, Ye W, Zhu Y, Cui M, Zhou J, Xiao C, et al. USP1/UAF1-stabilized METTL3 promotes reactive astrogliosis and improves functional recovery after spinal cord injury through m(6)A modification of YAP1 mRNA. J Neurosci. 2023;43:1456–74.
Rennie ML, Arkinson C, Chaugule VK, Toth R, Walden H. Structural basis of FANCD2 deubiquitination by USP1-UAF1. Nat Struct Mol Biol. 2021;28:356–64.
Han D, Wang L, Chen B, Zhao W, Liang Y, Li Y, et al. USP1-WDR48 deubiquitinase complex enhances TGF-β induced epithelial-mesenchymal transition of TNBC cells via stabilizing TAK1. Cell Cycle. 2021;20:320–31.
Pan W, Zhu S, Qu K, Meeth K, Cheng J, He K, et al. The DNA methylcytosine dioxygenase Tet2 sustains immunosuppressive function of tumor-infiltrating myeloid cells to promote melanoma progression. Immunity. 2017;47:284–97.e5.
Yin H, Zhang X, Yang P, Zhang X, Peng Y, Li D, et al. RNA m6A methylation orchestrates cancer growth and metastasis via macrophage reprogramming. Nat Commun. 2021;12:1394.
Chen J, Xu X, Li Y, Li F, Zhang J, Xu Q, et al. Kdm6a suppresses the alternative activation of macrophages and impairs energy expenditure in obesity. Cell Death Differ. 2021;28:1688–704.
Han SJ, Sung N, Wang J, O’Malley BW, Lonard DM. Steroid receptor coactivator-3 inhibition generates breast cancer antitumor immune microenvironment. Breast Cancer Res. 2022;24:73.
Ewens A, Luo L, Berleth E, Alderfer J, Wollman R, Hafeez BB, et al. Doxorubicin plus interleukin-2 chemoimmunotherapy against breast cancer in mice. Cancer Res. 2006;66:5419–26.
Kasikara C, Davra V, Calianese D, Geng K, Spires TE, Quigley M, et al. Pan-TAM tyrosine kinase inhibitor BMS-777607 enhances anti-PD-1 mAb efficacy in a murine model of triple-negative breast cancer. Cancer Res. 2019;79:2669–83.
Abe S, Nagata H, Crosby EJ, Inoue Y, Kaneko K, Liu CX, et al. Combination of ultrasound-based mechanical disruption of tumor with immune checkpoint blockade modifies tumor microenvironment and augments systemic antitumor immunity. J Immunother Cancer. 2022;10:e003717.
David T, Mallavialle A, Faget J, Alcaraz LB, Lapierre M, du Roure PD, et al. Anti-cathepsin D immunotherapy triggers both innate and adaptive anti-tumour immunity in breast cancer. Br J Pharmacol. 2023. https://doi.org/10.1111/bph.16291.
Liang Q, Dexheimer TS, Zhang P, Rosenthal AS, Villamil MA, You C, et al. A selective USP1-UAF1 inhibitor links deubiquitination to DNA damage responses. Nat Chem Biol. 2014;10:298–304.
Olazabal-Herrero A, García-Santisteban I, Rodríguez JA. Mutations in the ‘Fingers’ subdomain of the deubiquitinase USP1 modulate its function and activity. FEBS J. 2016;283:929–46.
Zhu X, Wang P, Zhan X, Zhang Y, Sheng J, He S, et al. USP1-regulated reciprocal differentiation of Th17 cells and Treg cells by deubiquitinating and stabilizing TAZ. Cell Mol Immunol. 2023;20:252–63.
Christofides A, Strauss L, Yeo A, Cao C, Charest A, Boussiotis VA. The complex role of tumor-infiltrating macrophages. Nat Immunol. 2022;23:1148–56.
Yang Q, Guo N, Zhou Y, Chen J, Wei Q, Han M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm Sin B. 2020;10:2156–70.
Mo J, Liang H, Su C, Li P, Chen J, Zhang B. DDX3X: structure, physiologic functions and cancer. Mol Cancer. 2021;20:38.
Gringhuis SI, Hertoghs N, Kaptein TM, Zijlstra-Willems EM, Sarrami-Forooshani R, Sprokholt JK, et al. HIV-1 blocks the signaling adaptor MAVS to evade antiviral host defense after sensing of abortive HIV-1 RNA by the host helicase DDX3. Nat Immunol. 2017;18:225–35.
Tantravedi S, Vesuna F, Winnard PT Jr., Van Voss MRH, Van Diest PJ, Raman V. Role of DDX3 in the pathogenesis of inflammatory bowel disease. Oncotarget. 2017;8:115280–9.
Yao L, Hao Q, Wang M, Chen Y, Cao H, Zhang Q, et al. KLHL29-mediated DDX3X degradation promotes chemosensitivity by abrogating cell cycle checkpoint in triple-negative breast cancer. Oncogene. 2023;42:3514–28.
Zhang Q, Zheng L, Bai Y, Su C, Che Y, Xu J, et al. ITPR1-AS1 promotes small cell lung cancer metastasis by facilitating P21(HRAS) splicing and stabilizing DDX3X to activate the cRaf-MEK-ERK cascade. Cancer Lett. 2023;577:216426.
Gadek M, Sherr EH, Floor SN. The variant landscape and function of DDX3X in cancer and neurodevelopmental disorders. Trends Mol Med. 2023;29:726–39.
Wang W, Jia M, Zhao C, Yu Z, Song H, Qin Y, et al. RNF39 mediates K48-linked ubiquitination of DDX3X and inhibits RLR-dependent antiviral immunity. Sci Adv. 2021;7:eabe5877.
Liu F, Zhuang W, Song B, Yang Y, Liu J, Zheng Y, et al. MAVS-loaded unanchored Lys63-linked polyubiquitin chains activate the RIG-I-MAVS signaling cascade. Cell Mol Immunol. 2023;20:1186–202.
Song H, Zhao C, Yu Z, Li Q, Yan R, Qin Y, et al. UAF1 deubiquitinase complexes facilitate NLRP3 inflammasome activation by promoting NLRP3 expression. Nat Commun. 2020;11:6042.
Han D, Wang L, Jiang S, Yang Q. The ubiquitin-proteasome system in breast cancer. Trends Mol Med. 2023;29:599–621.
Kerneur C, Cano CE, Olive D. Major pathways involved in macrophage polarization in cancer. Front Immunol. 2022;13:1026954.
Perfetto M, Xu X, Lu C, Shi Y, Yousaf N, Li J, et al. The RNA helicase DDX3 induces neural crest by promoting AKT activity. Development. 2021;148:dev184341.
Lim KS, Li H, Roberts EA, Gaudiano EF, Clairmont C, Sambel LA, et al. USP1 is required for replication fork protection in BRCA1-deficient tumors. Mol Cell. 2018;72:925–41.e4.
Yu Z, Tong L, Ma C, Song H, Wang J, Chai L, et al. The UAF1-USP1 deubiquitinase complex stabilizes cGAS and facilitates antiviral responses. J Immunol. 2024;212:295–301.
Ma L, Lin K, Chang G, Chen Y, Yue C, Guo Q, et al. Aberrant activation of β-catenin signaling drives glioma tumorigenesis via USP1-mediated stabilization of EZH2. Cancer Res. 2019;79:72–85.
Woo SM, Kim S, Seo SU, Kim S, Park JW, Kim G, et al. Inhibition of USP1 enhances anticancer drugs-induced cancer cell death through downregulation of survivin and miR-216a-5p-mediated upregulation of DR5. Cell Death Dis. 2022;13:821.
Zhao Y, Xue C, Xie Z, Ouyang X, Li L. Comprehensive analysis of ubiquitin-specific protease 1 reveals its importance in hepatocellular carcinoma. Cell Prolif. 2020;53:e12908.
Chen HH, Yu HI, Rudy R, Lim SL, Chen YF, Wu SH, et al. DDX3 modulates the tumor microenvironment via its role in endoplasmic reticulum-associated translation. iScience. 2021;24:103086.
He X, Li T, Qin K, Luo S, Li Z, Ji Q, et al. Demalonylation of DDX3 by Sirtuin 5 promotes antiviral innate immune responses. Theranostics. 2021;11:7235–46.
Samir P, Kesavardhana S, Patmore DM, Gingras S, Malireddi RKS, Karki R, et al. DDX3X acts as a live-or-die checkpoint in stressed cells by regulating NLRP3 inflammasome. Nature. 2019;573:590–4.
Ku YC, Lai MH, Lo CC, Cheng YC, Qiu JT, Tarn WY, et al. DDX3 participates in translational control of inflammation induced by infections and injuries. Mol Cell Biol. 2018;39:e00285-18.
Chen Y, Wu Y, Guo L, Yuan S, Sun J, Zhao K, et al. Exosomal Lnc NEAT1 from endothelial cells promote bone regeneration by regulating macrophage polarization via DDX3X/NLRP3 axis. J Nanobiotechnol. 2023;21:98.
Kienes I, Bauer S, Gottschild C, Mirza N, Pfannstiel J, Schröder M, et al. DDX3X links NLRP11 to the regulation of type I interferon responses and NLRP3 inflammasome activation. Front Immunol. 2021;12:653883.
Chen D, Nemazanyy I, Peulen O, Shostak K, Xu X, Tang SC, et al. Elp3-mediated codon-dependent translation promotes mTORC2 activation and regulates macrophage polarization. EMBO J. 2022;41:e109353.
Luo T, Yang S, Zhao T, Zhu H, Chen C, Shi X, et al. Hepatocyte DDX3X protects against drug-induced acute liver injury via controlling stress granule formation and oxidative stress. Cell Death Dis. 2023;14:400.
Cheng P, Li S, Chen H. Macrophages in lung injury, repair, and fibrosis. Cells. 2021;10:436.
Luo L, Wang S, Hu Y, Wang L, Jiang X, Zhang J, et al. Precisely regulating M2 subtype macrophages for renal fibrosis resolution. ACS Nano. 2023;17:22508–26.
Verhoeven Y, Tilborghs S, Jacobs J, De Waele J, Quatannens D, Deben C, et al. The potential and controversy of targeting STAT family members in cancer. Semin Cancer Biol. 2020;60:41–56.
He S, Gao X, Yang L, Li X, Mo Y, He Z, et al. MiR-144/451 attenuates lipopolysaccharide-induced lung inflammation by downregulating Rac1 and STAT-3 in macrophages. J Biochem Mol Toxicol. 2024;38:e70006.
Juncadella IJ, Kadl A, Sharma AK, Shim YM, Hochreiter-Hufford A, Borish L, et al. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature. 2013;493:547–51.
Zhang Y, Zhang H, Zhao S, Qi Z, He Y, Zhang X, et al. S-nitrosylation of septin2 exacerbates aortic aneurysm and dissection by coupling the TIAM1-RAC1 axis in macrophages. Circulation. 2024;149:1903–20.
Spinelli FR, Colbert RA, Gadina M. JAK1: Number one in the family; number one in inflammation?. Rheumatology. 2021;60:ii3–ii10.
He L, Tian L. Downregulation of miR‑409‑3p suppresses LPS‑induced inflammation in human bronchial epithelial cells through SOCS3/JAK1/STAT3 signaling: the implication for bronchopneumonia. Mol Med Rep. 2021;23:190.
Kubo S, Nakayamada S, Tanaka Y. JAK inhibitors for rheumatoid arthritis. Expert Opin Investig Drugs. 2023;32:333–44.
Fayand A, Hentgen V, Posseme C, Lacout C, Picard C, Moguelet P, et al. Successful treatment of JAK1-associated inflammatory disease. J Allergy Clin Immunol. 2023;152:972–83.
Shi Y, Bollam SR, White SM, Laughlin SZ, Graham GT, Wadhwa M, et al. Rac1-mediated DNA damage and inflammation promote Nf2 tumorigenesis but also limit cell-cycle progression. Dev Cell. 2016;39:452–65.
Martínez-Sánchez LDC, Ngo PA, Pradhan R, Becker LS, Boehringer D, Soteriou D, et al. Epithelial RAC1-dependent cytoskeleton dynamics controls cell mechanics, cell shedding and barrier integrity in intestinal inflammation. Gut. 2023;72:275–94.
Liu Y, Zhang Y, Wang C, Liu Q, Li T, Wang W, et al. Inhibition of DDX3X alleviates persistent inflammation, immune suppression and catabolism syndrome in a septic mice model. Int Immunopharmacol. 2023;117:109779.
Liu Y, Zhang Y, Liu Q, Li T, Wang W, Li H, et al. Inhibition of DDX3X ameliorated CD4(+) T cells pyroptosis and improves survival in septic mice. Mol Immunol. 2023;154:54–60.
Hofmann C, Serafin A, Schwerdt OM, Fischer J, Sicklinger F, Younesi FS, et al. Transient inhibition of translation improves cardiac function after ischemia/reperfusion by attenuating the inflammatory response. Circulation. 2024;150:1248–67.
Anderson P. Post-transcriptional regulons coordinate the initiation and resolution of inflammation. Nat Rev Immunol. 2010;10:24–35.
Jia X, Yan B, Tian X, Liu Q, Jin J, Shi J, et al. CD47/SIRPα pathway mediates cancer immune escape and immunotherapy. Int J Biol Sci. 2021;17:3281–7.
Wang L, Zhang N, Han D, Su P, Chen B, Zhao W, et al. MTDH promotes intestinal inflammation by positively regulating TLR signalling. J Crohns Colitis. 2021;15:2103–17.
Han D, Wang L, Long L, Su P, Luo D, Zhang H, et al. The E3 ligase TRIM4 facilitates SET ubiquitin-mediated degradation to enhance ER-α action in breast cancer. Adv Sci. 2022;9:e2201701.
Funding
This research was supported by National Key Research and Development Program (No. 2020YFA0712400), Special Foundation for Taishan Scholars (No. ts20190971), Special Support Plan for National High Level Talents (Ten Thousand Talents Program W01020103), Foundation from Clinical Research Center of Shandong University (No.2020SDUCRCA015), Qilu Hospital Clinical New Technology Developing Foundation (No. 2019-3) to QY; National Natural Science Foundation of China (No. 82171734), the Shandong Provincial Natural Science Foundation (No. ZR2021MH048) to LW; National Natural Science Foundation of China (No. 82403732), China Postdoctoral Science Foundation (2023M742108), Shandong Postdoctoral Science Foundation (SDCX-ZG-202400073) to DH.
Author information
Authors and Affiliations
Contributions
QY designed research studies. DH, LW, and SJ performed most of the experiments in this work. PS collected patient samples. TC and DL analyzed data. BC, WZ, NZ, XW, YRL, and YML provided valuable discussion. LW and DH wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Breast cancer tissue specimens were collected from Qilu Hospital of Shandong University. This study was approved by the Research Ethics Committee of Qilu Hospital of Shandong University (KYLL-2016-255), with all participants providing informed consent and ensuring strict privacy protection. Animal experiments complied with China’s Guidelines for Animal Health and Use and were approved by the Ethics Committee of Qilu Hospital of Shandong University (KYLL-2024(ZM)-436).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, D., Wang, L., Jiang, S. et al. The USP1-WDR48 deubiquitinase complex functions as a molecular switch regulating tumor-associated macrophage activation and anti-tumor response. Cell Death Differ (2025). https://doi.org/10.1038/s41418-025-01548-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41418-025-01548-x