Abstract
Metastasis significantly contributes to the high mortality of non-small cell lung cancer (NSCLC), with anoikis resistance playing a critical role in this process. However, the role of TRIM32 in anoikis resistance and metastasis is not well understood. In this study, we demonstrate that TRIM32 enhances both anoikis resistance and metastasis in NSCLC. We confirmed the interaction between TRIM32 and CHEK2, and showed that TRIM32 mediates the degradation of CHEK2 via K48-linked polyubiquitination. Our results indicate that CHEK2 suppresses anoikis resistance and metastasis in NSCLC, both in vitro and in vivo. Additionally, we found that TRIM32 upregulates IL-6 expression, an effect that is reversed by the overexpression of CHEK2. Further analysis confirmed that IL-6 plays a key role in TRIM32-mediated anoikis resistance and metastasis. Notably, TRIM32+CHEK2-IL-6+ tumor cells were more prevalent in NSCLC tissues with lymph node metastasis. In conclusion, our findings suggest that targeting TRIM32 to inhibit anoikis resistance and metastasis, via the CHEK2/IL-6 axis, may provide a novel therapeutic strategy for treating metastatic NSCLC.

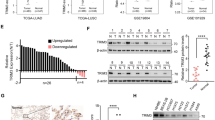
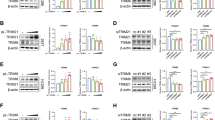
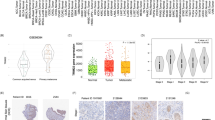
The mechanisms by which TRIM32 promotes anoikis resistance and metastasis in NSCLC. Schematic diagram showing the regulatory mechanisms of TRIM32 promotes anoikis resistance and metastasis in NSCLC by degrading CHEK2 to promote IL-6 secretion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J Clin. 2021;71:209–49.
Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet. 2021;398:535–54.
Wang C, Wu Y, Shao J, Liu D, Li W. Clinicopathological variables influencing overall survival, recurrence and post-recurrence survival in resected stage I non-small-cell lung cancer. BMC Cancer. 2020;20:150.
Klein CA. Cancer progression and the invisible phase of metastatic colonization. Nat Rev Cancer. 2020;20:681–94.
Xie S, Wu Z, Qi Y, Wu B, Zhu X. The metastasizing mechanisms of lung cancer: recent advances and therapeutic challenges. Biomed Pharmacother. 2021;138:111450.
Taddei ML, Giannoni E, Fiaschi T, Chiarugi P. Anoikis: an emerging hallmark in health and diseases. J Pathol. 2012;226:380–93.
Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–6.
Simpson CD, Anyiwe K, Schimmer AD. Anoikis resistance and tumor metastasis. Cancer Lett. 2008;272:177–85.
Zhan W, Zhang S. TRIM proteins in lung cancer: mechanisms, biomarkers and therapeutic targets. Life Sci. 2021;268:118985.
Bawa S, Piccirillo R, Geisbrecht ER. TRIM32: a multifunctional protein involved in muscle homeostasis, glucose metabolism, and tumorigenesis. Biomolecules. 2021;11:408.
Lazzari E, Meroni G. TRIM32 ubiquitin E3 ligase, one enzyme for several pathologies: from muscular dystrophy to tumours. Int J Biochem Cell B. 2016;79:469–77.
Romagnoli A, Di Rienzo M, Petruccioli E, Fusco C, Palucci I, Micale L, et al. The ubiquitin ligase TRIM32 promotes the autophagic response to Mycobacterium tuberculosis infection in macrophages. Cell Death Dis. 2023;14:505.
Chen Z, Tian L, Wang L, Ma X, Lei F, Chen X, et al. TRIM32 inhibition attenuates apoptosis, oxidative stress, and inflammatory injury in podocytes induced by high glucose by modulating the Akt/GSK-3beta/Nrf2 pathway. Inflammation. 2022;45:992–1006.
Di Rienzo M, Antonioli M, Fusco C, Liu Y, Mari M, Orhon I, et al. Autophagy induction in atrophic muscle cells requires ULK1 activation by TRIM32 through unanchored K63-linked polyubiquitin chains. Sci Adv. 2019;5:u8857.
Wang C, Xu J, Fu H, Zhang Y, Zhang X, Yang D, et al. TRIM32 promotes cell proliferation and invasion by activating beta-catenin signalling in gastric cancer. J Cell Mol Med. 2018;22:5020–8.
Kano S, Miyajima N, Fukuda S, Hatakeyama S. Tripartite motif protein 32 facilitates cell growth and migration via degradation of Abl-interactor 2. Cancer Res. 2008;68:5572–80.
Huang C. Roles of E3 ubiquitin ligases in cell adhesion and migration. Cell Adhes Migr. 2010;4:10–8.
Chen F, Guo Q, Chen Q, Han Z, Zhou X, Wu L, et al. TRIM32 triggers beta-catenin signaling through ubiquitylation of AXIN1 to promote inflammatory factor-induced apoptosis of rat nucleus pulposus cells. AM J Physiol Cell PH. 2020;318:C695–703.
Propper DJ, Balkwill FR. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol. 2022;19:237–53.
Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Perez-Gracia JL, et al. Cytokines in clinical cancer immunotherapy. Brit J Cancer. 2019;120:6–15.
Kumari N, Dwarakanath BS, Das A, Bhatt AN. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37:11553–72.
Boonen R, Vreeswijk M, van Attikum H. CHEK2 variants: linking functional impact to cancer risk. Trends Cancer. 2022;8:759–70.
Spachmann PJ, Azzolina V, Weber F, Evert M, Eckstein M, Denzinger S, et al. Loss of CHEK2 predicts progression in stage pT1 non-muscle-invasive bladder cancer (NMIBC). Pathol Oncol Res. 2020;26:1625–32.
Suchy J, Cybulski C, Wokolorczyk D, Oszurek O, Gorski B, Debniak T, et al. CHEK2 mutations and HNPCC-related colorectal cancer. Int J Cancer. 2010;126:3005–9.
Stolarova L, Kleiblova P, Janatova M, Soukupova J, Zemankova P, Macurek L, et al. CHEK2 germline variants in cancer predisposition: stalemate rather than checkmate. Cells. 2020;9:2675.
Hu HF, Han L, Fu JY, He X, Tan JF, Chen QP, et al. LINC00982-encoded protein PRDM16-DT regulates CHEK2 splicing to suppress colorectal cancer metastasis and chemoresistance. Theranostics. 2024;14:3317–38.
Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Csh Perspect Biol. 2014;6:a16295.
Tanaka T, Narazaki M, Kishimoto T. Interleukin (IL-6) Immunotherapy. Csh Perspect Biol. 2018;10:a028456.
Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–57.
Wang L, Cao L, Wang H, Liu B, Zhang Q, Meng Z, et al. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget. 2017;8:76116–28.
Al-Rakan MA, Hendrayani SF, Aboussekhra A. CHEK2 represses breast stromal fibroblasts and their paracrine tumor-promoting effects through suppressing SDF-1 and IL-6. BMC Cancer. 2016;16:575.
Acknowledgements
We sincerely thank Prof. Dongming Kuang from Sun Yat-sen University for his invaluable guidance and support, which were instrumental in the successful completion of this research. His insightful suggestions and expertise provided critical contributions to the study’s direction and outcomes. We also appreciate the assistance and constructive feedback from our colleagues and collaborators, which significantly enhanced the quality of this work.
Funding
This research was funded by Key Project of Jiangsu Provincial Health Commission (zd2021050), State-level Key Laboratory of Clinical Immunology (Soochow University) (9012280224), Su Zhou Basic Research Pilot Project (SSD2024098), Suzhou Applied Basic research (SKY2022128), National Natural Science Foundation of China (82303146,81872328,81372276,30901789), 2024 Reserve Talent Program (0499980201048, 0302020201048).
Author information
Authors and Affiliations
Contributions
Jia Xu, Conceptualisation, Validation, Data Management, Formal Analysis, Writing Originals, Visualisation. Wenjun Liu, Conceptualisation, Formal Analysis, Data Management, Validation. Li Chen, Conceptualisation, Software. Juan Zhang, Software, Investigation. Dongze Zhang, Conceptualisation, Investigation. Xue Huang, Funding Acquisition, Methodology, Project Management, Writing-Review and Editing, Supervision. Guangbo Zhang, Funding Acquisition, Methodology, Project Management, Writing-Review and Editing, Resources.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
All animal experiments were conducted according to the guidelines of the First Affiliated Hospital of Soochow University and approved by the Laboratory Animal Ethics Committee of Soochow University. The present study was approved by the Bioscience Ethics Review Committee of Hunan Aifang Biotechnology Co., Ltd., and all enrolled patients provided written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, J., Liu, W., Chen, L. et al. TRIM32 promotes anoikis resistance and metastasis in NSCLC by degrading CHEK2 to enhance IL-6 secretion. Cell Death Differ (2025). https://doi.org/10.1038/s41418-025-01559-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41418-025-01559-8