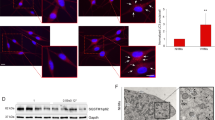
Abstract
NLRP3 functions as a critical intracellular danger sensor for inflammasome activation, playing a crucial role in autoimmune diseases. Vitiligo progression has been linked to NLRP3, yet its specific involvement in melanocytes of vitiligo remains poorly understood. In this study, we demonstrate that NLRP3 expression is significantly upregulated in the melanocytes of vitiligo patients and melanoma-Treg-induced vitiligo mouse model. Genetic knockout of NLRP3 effectively alleviates vitiligo progression in these mice. Our mechanistic investigations reveal that the downregulation of the E3 ligase β-TrCP1 in vitiligo melanocytes decreases K27-linked ubiquitination levels of NLRP3, which in turn weakens its interaction with the autophagy receptor NDP52. This disruption impairs the selective autophagic degradation of NLRP3, leading to hyperactivation of inflammation and pyroptosis in melanocytes, thereby accelerating vitiligo pathogenesis. Notably, melanocyte-specific knockdown of NLRP3 using lysine-proline-valine (KPV)-modified deformable liposomes (KPV-Lipos) carrying Nlrp3 shRNA significantly alleviates vitiligo development. This study elucidates the mechanism by which autophagy dysfunction mediated excessive NLRP3 inflammasome activation in melanocytes contributes to vitiligo pathogenesis, highlighting potential therapeutic strategies targeting these pathways for the treatment of vitiligo and other pigment-related skin diseases.

Overview of disrupted NLRP3 autophagic degradation in vitiligo melanocytes. In healthy melanocytes, NLRP3 expression is upregulated when subjected to oxidative stress, along with an increase in the E3 ligase β-TrCP1, which enhances the K27-linked ubiquitination of NLRP3 and further strengthens its binding to the autophagy receptor protein NDP52, thus effectively suppressing the excessive inflammatory response. Whereas in the melanocytes of vitiligo patients, decreased expression of β-TrCP1 leads to downregulation of K27-linked ubiquitination in NLRP3, thus inhibiting its autophagic degradation. The persistent activation of NLRP3 in vitiligo melanocytes promotes the cleavage of pro-IL-1β and GSDMD. GSDMD-N subsequently forms pores on the cell membrane, which causes the release of IL-1β and results in melanocyte pyroptosis. In our study, we utilize KPV-Lipos with Nlrp3 shRNA to precisely knockdown NLRP3 expression in melanocytes and effectively alleviate vitiligo development, which provide a potentially promising strategy for the treatment of vitiligo. MC, melanocytes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
We declare that all the data and materials are available upon request.
References
Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet. 2015;386:74–84.
Zhang B, Ma S, Rachmin I, He M, Baral P, Choi S, et al. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature. 2020;577:676–81.
Xu Z, Chen D, Hu Y, Jiang K, Huang H, Du Y, et al. Anatomically distinct fibroblast subsets determine skin autoimmune patterns. Nature. 2021;601:118–24.
van den Boorn JG, Konijnenberg D, Dellemijn TA, van der Veen JP, Bos JD, Melief CJ, et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol. 2009;129:2220–32.
Malik BT, Byrne KT, Vella JL, Zhang P, Shabaneh TB, Steinberg SM, et al. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci Immunol. 2017;2:eaam6346.
Cheuk S, Schlums H, Gallais Serezal I, Martini E, Chiang SC, Marquardt N, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity. 2017;46:287–300.
Boniface K, Jacquemin C, Darrigade AS, Dessarthe B, Martins C, Boukhedouni N, et al. Vitiligo skin is imprinted with resident memory CD8 T cells expressing CXCR3. J Invest Dermatol. 2018;138:355–64.
Gellatly KJ, Strassner JP, Essien K, Refat MA, Murphy RL, Coffin-Schmitt A, et al. scRNA-seq of human vitiligo reveals complex networks of subclinical immune activation and a role for CCR5 in T(reg) function. Sci Transl Med. 2021;13:eabd8995.
Hsueh YC, Wang Y, Riding RL, Catalano DE, Lu YJ, Richmond JM, et al. A keratinocyte-tethered biologic enables location-precise treatment in mouse vitiligo. J Invest Dermatol. 2022;142:3294–303.
Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15:713–20.
Klionsky DJ, Petroni G, Amaravadi RK, Baehrecke EH, Ballabio A, Boya P, et al. Autophagy in major human diseases. Embo j. 2021;40:e108863.
Qiao Z, Wang X, Xiang L, Zhang C. Dysfunction of autophagy: a possible mechanism involved in the pathogenesis of vitiligo by breaking the redox balance of melanocytes. Oxid Med Cell Longev. 2016;2016:3401570.
Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, et al. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997;275:206–9.
Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–74.
Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–87.
Tang J, Tu S, Lin G, Guo H, Yan C, Liu Q, et al. Sequential ubiquitination of NLRP3 by RNF125 and Cbl-b limits inflammasome activation and endotoxemia. J Exp Med. 2020;217:e20182091.
Song H, Liu B, Huai W, Yu Z, Wang W, Zhao J, et al. The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3. Nat Commun. 2016;7:13727.
Han S, Lear TB, Jerome JA, Rajbhandari S, Snavely CA, Gulick DL, et al. Lipopolysaccharide primes the NALP3 inflammasome by inhibiting its ubiquitination and degradation mediated by the SCFFBXL2 E3 Ligase. J Biol Chem. 2015;290:18124–33.
Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015;160:62–73.
Li S, Kang P, Zhang W, Jian Z, Zhang Q, Yi X, et al. Activated NLR family pyrin domain containing 3 (NLRP3) inflammasome in keratinocytes promotes cutaneous T-cell response in patients with vitiligo. J Allergy Clin Immunol. 2020;145:632–45.
Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411.
Zhu F, Ma J, Li W, Liu Q, Qin X, Qian Y, et al. The orphan receptor Nur77 binds cytoplasmic LPS to activate the non-canonical NLRP3 inflammasome. Immunity. 2023;56:753–67.e8.
Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–91.
Christgen S, Place DE, Kanneganti TD. Toward targeting inflammasomes: insights into their regulation and activation. Cell Res. 2020;30:315–27.
He Y, Li S, Zhang W, Dai W, Cui T, Wang G, et al. Dysregulated autophagy increased melanocyte sensitivity to H(2)O(2)-induced oxidative stress in vitiligo. Sci Rep. 2017;7:42394.
Cosin-Roger J, Simmen S, Melhem H, Atrott K, Frey-Wagner I, Hausmann M, et al. Hypoxia ameliorates intestinal inflammation through NLRP3/mTOR downregulation and autophagy activation. Nat Commun. 2017;8:98.
Vargas JNS, Hamasaki M, Kawabata T, Youle RJ, Yoshimori T. The mechanisms and roles of selective autophagy in mammals. Nat Rev Mol Cell Biol. 2023;24:167–85.
Liu T, Tang Q, Liu K, Xie W, Liu X, Wang H, et al. TRIM11 suppresses AIM2 inflammasome by degrading AIM2 via p62-dependent selective autophagy. Cell Rep. 2016;16:1988–2002.
Zheng N, Shabek N. Ubiquitin ligases: structure, function, and regulation. Annu Rev Biochem. 2017;86:129–57.
Akther M, Haque ME, Park J, Kang TB, Lee KH. NLRP3 ubiquitination-a new approach to target NLRP3 inflammasome activation. Int J Mol Sci 2021;22:8780.
Sun MC, Xu XL, Du Y, Lou XF, Wang W, You YC, et al. Biomimetic melanosomes promote orientation-selective delivery and melanocyte pigmentation in the H(2)O(2)-induced vitiligo mouse model. ACS Nano. 2021;15:17361–74.
Cevc G. Transfersomes, liposomes and other lipid suspensions on the skin: permeation enhancement, vesicle penetration, and transdermal drug delivery. Crit Rev Ther Drug Carr Syst. 1996;13:257–388.
Wooff Y, Man SM, Aggio-Bruce R, Natoli R, Fernando N. IL-1 Family members mediate cell death, inflammation and angiogenesis in retinal degenerative diseases. Front Immunol. 2019;10:1618.
Zhang Z, Li M, Li X, Feng Z, Luo G, Wang Y, et al. Glutamine metabolism modulates microglial NLRP3 inflammasome activity through mitophagy in Alzheimer’s disease. J Neuroinflam. 2024;21:261.
Jiang H, Xie Y, Hu Z, Lu J, Zhang J, Li H, et al. VANGL2 alleviates inflammatory bowel disease by recruiting the ubiquitin ligase MARCH8 to limit NLRP3 inflammasome activation through OPTN-mediated selective autophagy. PLoS Biol. 2025;23:e3002961.
Yang S, Li M, Lian G, Wu Y, Cui J, Wang L. ABHD8 antagonizes inflammation by facilitating chaperone-mediated autophagy-mediated degradation of NLRP3. Autophagy. 2025;21:338–51.
Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18:2114–27.
Li LR, Chen L, Sun ZJ. Igniting hope: Harnessing NLRP3 inflammasome-GSDMD-mediated pyroptosis for cancer immunotherapy. Life Sci. 2024;354:122951.
Xu T, Yu W, Fang H, Wang Z, Chi Z, Guo X, et al. Ubiquitination of NLRP3 by gp78/Insig-1 restrains NLRP3 inflammasome activation. Cell Death Differ. 2022;29:1582–95.
Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell. 2013;49:331–8.
Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri ES. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem. 2012;287:36617–22.
Nie L, Fei C, Fan Y, Dang F, Zhao Z, Zhu T, et al. Consecutive palmitoylation and phosphorylation orchestrates NLRP3 membrane trafficking and inflammasome activation. Mol Cell. 2024;84:3336–53.e7.
Qin Y, Zhao W. Posttranslational modifications of NLRP3 and their regulatory roles in inflammasome activation. Eur J Immunol 2023;53:e2350382.
Jing J, Yang F, Wang K, Cui M, Kong N, Wang S, et al. UFMylation of NLRP3 prevents its autophagic degradation and facilitates inflammasome activation. Adv Sci. 2025;12:e2406786.
Sosič I, Bricelj A, Steinebach C. E3 ligase ligand chemistries: from building blocks to protein degraders. Chem Soc Rev. 2022;51:3487–534.
Hang Y, Tan L, Chen Q, Liu Q, Jin Y. E3 ubiquitin ligase TRIM24 deficiency promotes NLRP3/caspase-1/IL-1β-mediated pyroptosis in endometriosis. Cell Biol Int. 2021;45:1561–70.
Shen J, Wu Q, Liang T, Zhang J, Bai J, Yuan M, et al. TRIM40 inhibits IgA1-induced proliferation of glomerular mesangial cells by inactivating NLRP3 inflammasome through ubiquitination. Mol Immunol. 2021;140:225–32.
Humphries F, Bergin R, Jackson R, Delagic N, Wang B, Yang S, et al. The E3 ubiquitin ligase Pellino2 mediates priming of the NLRP3 inflammasome. Nature Communications 2018;9:1560.
Wang D, Zhang Y, Xu X, Wu J, Peng Y, Li J, et al. YAP promotes the activation of NLRP3 inflammasome via blocking K27-linked polyubiquitination of NLRP3. Nat Commun. 2021;12:2674.
He Y, Jiang X, Duan L, Xiong Q, Yuan Y, Liu P, et al. LncRNA PKMYT1AR promotes cancer stem cell maintenance in non-small cell lung cancer via activating Wnt signaling pathway. Mol Cancer. 2021;20:156.
Xie W, Jiang Q, Wu X, Wang L, Gao B, Sun Z, et al. IKBKE phosphorylates and stabilizes Snail to promote breast cancer invasion and metastasis. Cell Death Differ. 2022;29:1528–40.
Zhu Y, Qu C, Hong X, Jia Y, Lin M, Luo Y, et al. Trabid inhibits hepatocellular carcinoma growth and metastasis by cleaving RNF8-induced K63 ubiquitination of Twist1. Cell Death Differ. 2019;26:306–20.
Fuchs SY, Spiegelman VS, Kumar KG. The many faces of beta-TrCP E3 ubiquitin ligases: reflections in the magic mirror of cancer. Oncogene. 2004;23:2028–36.
Bi Y, Cui D, Xiong X, Zhao Y. The characteristics and roles of beta-TrCP1/2 in carcinogenesis. FEBS J. 2021;288:3351–74.
Song H, Zhao C, Yu Z, Li Q, Yan R, Qin Y, et al. UAF1 deubiquitinase complexes facilitate NLRP3 inflammasome activation by promoting NLRP3 expression. Nat Commun. 2020;11:6042.
Murai J, Yang K, Dejsuphong D, Hirota K, Takeda S, D’Andrea AD. The USP1/UAF1 complex promotes double-strand break repair through homologous recombination. Mol Cell Biol. 2011;31:2462–9.
Palazón-Riquelme P, Worboys JD, Green J, Valera A, Martín-Sánchez F, Pellegrini C, et al. USP7 and USP47 deubiquitinases regulate NLRP3 inflammasome activation. EMBO Rep 2018;19:e44766.
Cai B, Zhao J, Zhang Y, Liu Y, Ma C, Yi F, et al. USP5 attenuates NLRP3 inflammasome activation by promoting autophagic degradation of NLRP3. Autophagy. 2022;18:990–1004.
Hai B, Mao T, Du C, Jia F, Liu Y, Song Q, et al. USP14 promotes pyroptosis of human annulus fibrosus cells derived from patients with intervertebral disc degeneration through deubiquitination of NLRP3. Acta Biochim Biophys Sin. 2022;54:1–11.
Kocak M, Ezazi Erdi S, Jorba G, Maestro I, Farres J, Kirkin V, et al. Targeting autophagy in disease: established and new strategies. Autophagy. 2022;18:473–95.
Cui T, Wang Y, Song P, Yi X, Chen J, Yang Y, et al. HSF1-dependent autophagy activation contributes to the survival of melanocytes under oxidative stress in vitiligo. J Invest Dermatol. 2022;142:1659–69.e4.
Qiao Z, Xu Z, Xiao Q, Yang Y, Ying J, Xiang L, et al. Dysfunction of ATG7-dependent autophagy dysregulates the antioxidant response and contributes to oxidative stress-induced biological impairments in human epidermal melanocytes. Cell Death Discov. 2020;6:31.
Zhang D, Zhang Y, Pan J, Cao J, Sun X, Li X, et al. Degradation of NLRP3 by p62-dependent-autophagy improves cognitive function in Alzheimer’s disease by maintaining the phagocytic function of microglia. CNS Neurosci Ther 2023;29:2826–42.
Wang Y, Li S, Li C. Clinical features, immunopathogenesis, and therapeutic strategies in vitiligo. Clin Rev Allergy Immunol. 2021;61:299–323.
Richmond JM, Bangari DS, Essien KI, Currimbhoy SD, Groom JR, Pandya AG, et al. Keratinocyte-derived chemokines orchestrate T-cell positioning in the epidermis during vitiligo and may serve as biomarkers of disease. J Invest Dermatol. 2017;137:350–8.
Xie B, Song X. The impaired unfolded protein-premelanosome protein and transient receptor potential channels-autophagy axes in apoptotic melanocytes in vitiligo. Pigment Cell Melanoma Res. 2022;35:6–17.
Felsten LM, Alikhan A, Petronic-Rosic V. Vitiligo: a comprehensive overview Part II: treatment options and approach to treatment. J Am Acad Dermatol. 2011;65:493–514.
Whitton ME, Pinart M, Batchelor J, Leonardi-Bee J, González U, Jiyad Z, et al. Interventions for vitiligo. Cochrane Database Syst Rev 2015;2015:Cd003263.
Speeckaert R, van Geel N. Vitiligo: an update on pathophysiology and treatment options. Am J Clin Dermatol. 2017;18:733–44.
Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–9.
Le Gal FA, Avril MF, Bosq J, Lefebvre P, Deschemin JC, Andrieu M, et al. Direct evidence to support the role of antigen-specific CD8(+) T cells in melanoma-associated vitiligo. J Invest Dermatol. 2001;117:1464–70.
Acknowledgements
We thank Dr. Chunying Li (Xijing Hospital, Fourth Military Medical University) for providing PIG1 and PIG3V cells and Dr. Jun Cui (Sun Yat-sen University) for providing plasmids for p62, NDP52, Tollip and OPTN.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82572009, 82473549, 82371761, and 82171741), National Key R&D Program of China (2024YFC2309700), Guangdong Basic and Applied Basic Research Foundation (2023A1515010421), Science and Technology Program of Guangzhou (2025A04J7166), Zhejiang Provincial Natural Science Foundation of China (LY23H110001), and The Hangzhou Medical Key Discipline Construction Project ([2025]36-7).
Author information
Authors and Affiliations
Contributions
Ke Zeng, Yuqi Zhu and Zhongxin Han designed the study, performed the major experiments and analyzed data. Siyi Xiong, Yan Zhao, Zilong Xiao, Yingchao Xie, Weiwei Liu and Yongzhong Du provided technique support in some experiments. Ke Zeng, Yuqi Zhu and Zhongxin Han wrote the original manuscript. Xiao Yu and Cuiping Guan edited the manuscript. Shiyu Jin, Tingru Dong and Lan Lan performed some animal experiments. Xiao Yu, Cuiping Guan and Xiuzu Song supervised all experiments and verified the experimental data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. Animal (mice) procedures were approved by Southern Medical University Animal Care and Use Committee (Protocol number: L2019043). Human tissue collection and subsequent studies were approved by the ethics committee of Hangzhou Third People’s Hospital, Hangzhou, China, and informed consent was obtained from all patients prior to participation. (Protocol number: 2022KA007).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeng, K., Zhu, Y., Han, Z. et al. NLRP3 autophagic degradation disruption in melanocytes contributes to vitiligo development. Cell Death Differ 33, 343–357 (2026). https://doi.org/10.1038/s41418-025-01578-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-025-01578-5
This article is cited by
-
The role of TRIM proteins in the pathogenesis of mycobacterium tuberculosis
Biology Direct (2025)