Abstract
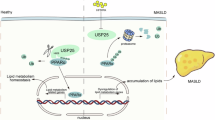
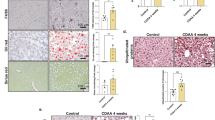
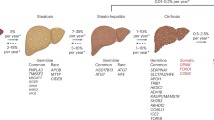
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a leading cause of chronic liver disease worldwide, yet the molecular mechanisms underlying its pathogenesis are not fully understood. Here, we identify the deubiquitinating enzyme Ubiquitin-specific protease 2 (USP2) as a key regulator in hepatic lipid metabolism and MASLD progression. We show that USP2 expression is significantly upregulated in liver tissues from MASLD patients and high-fat diet (HFD)-induced mouse models. Usp2 knockout or pharmacological inhibition alleviates hepatic steatosis and improves systemic metabolic parameters both in vivo and in vitro. Strikingly, hepatocyte-targeted GalNAc-conjugated siRNA against Usp2 markedly attenuates MASLD in mouse models, highlighting therapeutic potential. Mechanistically, USP2 directly interacts with and stabilizes peroxisome proliferator-activated receptor γ (PPARγ) by removing K48-linked ubiquitin chains at lysine 161 within its DNA-binding domain, thereby preventing proteasomal degradation and enhancing its transcriptional activity. This USP2-PPARγ axis promotes hepatic lipid accumulation and drives MASLD progression. Our findings uncover a novel regulatory mechanism in MASLD pathogenesis and suggest that USP2 may represent a promising and druggable therapeutic target for metabolic liver disease.
Highlights
-
USP2 expression is significantly upregulated in liver tissues from both MASLD patients and HFD-induced mouse models.
-
Hepatocyte-specific knockout of Usp2 protects mice from hepatic steatosis and improves metabolic parameters.
-
USP2 stabilizes PPARγ by removing K48-linked ubiquitin chains at lysine 161, thereby promoting lipid accumulation.
-
GalNAc-siRNAs targeting hepatic Usp2 offer a promising treatment strategy for MASLD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The raw RNA-sequencing data have been submitted to the National Center for Biotechnologyinformation (accession number: PRJNA1280771). All data are available from the corresponding authors upon request.
References
Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542–56.
Wong VW, Ekstedt M, Wong GL, Hagström H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023;79:842–52.
Francque SM, Marchesini G, Kautz A, Walmsley M, Dorner R, Lazarus JV, et al. Non-alcoholic fatty liver disease: A patient guideline. JHEP Rep. 2021;3:100322.
Allen AM, Younossi ZM, Diehl AM, Charlton MR, Lazarus JV. Envisioning how to advance the MASH field. Nat Rev Gastroenterol Hepatol. 2024;21:726–38.
Horn P, Tacke F. Metabolic reprogramming in liver fibrosis. Cell Metab. 2024;36:1439–55.
Zhang Y, Wang X, Lin J, Liu J, Wang K, Nie Q, et al. A microbial metabolite inhibits the HIF-2α-ceramide pathway to mediate the beneficial effects of time-restricted feeding on MASH. Cell Metab. 2024;36:1823–1838.e6-e6.
Zhang X, Zheng MH, Liu D, Lin Y, Song SJ, Chu ES, et al. A blood-based biomarker panel for non-invasive diagnosis of metabolic dysfunction-associated steatohepatitis. Cell Metab. 2025;37:59–68.e3.
Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–22.
Chui ZSW, Xue Y, Xu A. Hormone-based pharmacotherapy for metabolic dysfunction-associated fatty liver disease. Med Rev (2021). 2024;4:158–68.
Feng JN, Jin T. Hepatic function of glucagon-like peptide-1 and its based diabetes drugs. Med Rev (2021). 2024;4:312–25.
Keam SJ. Resmetirom: first approval. Drugs. 2024;84:729–35.
O’Leary K. Semaglutide treats MASH liver disease. Nat Med. 2025. https://doi.org/10.1038/d41591-025-00034-8.
Pouly D, Chenaux S, Martin V, Babis M, Koch R, Nagoshi E, et al. USP2-45 is a circadian clock output effector regulating calcium absorption at the post-translational level. PLoS One. 2016;11:e0145155.
Wang S, Nie J, Jiang H, Li A, Zhong N, Tong W, et al. VCP enhances autophagy-related osteosarcoma progression by recruiting USP2 to inhibit ubiquitination and degradation of FASN. Cell Death Dis. 2024;15:788.
Calvisi DF, Wang C, Ho C, Ladu S, Lee SA, Mattu S, et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140:1071–83.
Molusky MM, Li S, Ma D, Yu L, Lin JD. Ubiquitin-specific protease 2 regulates hepatic gluconeogenesis and diurnal glucose metabolism through 11β-hydroxysteroid dehydrogenase 1. Diabetes. 2012;61:1025–35.
Li C, Li M, Sheng W, Zhou W, Zhang Z, Ji G, et al. High dietary fructose drives metabolic dysfunction-associated steatotic liver disease via activating ubiquitin-specific peptidase 2/11β-hydroxysteroid dehydrogenase type 1 pathway in mice. Int J Biol Sci. 2024;20:3480–96.
Saito N, Kimura S, Miyamoto T, Fukushima S, Amagasa M, Shimamoto Y, et al. Macrophage ubiquitin-specific protease 2 modifies insulin sensitivity in obese mice. Biochem Biophys Rep. 2017;9:322–9.
Liu Q, Ma L, Chen F, Zhang S, Huang Z, Zheng X, et al. Raloxifene-driven benzothiophene derivatives: Discovery, structural refinement, and biological evaluation as potent PPARγ modulators based on drug repurposing. Eur J Med Chem. 2024;269:116325.
Staels B, Butruille L, Francque S. Treating NASH by targeting peroxisome proliferator-activated receptors. J Hepatol. 2023;79:1302–16.
Filali-Mouncef Y, Hunter C, Roccio F, Zagkou S, Dupont N, Primard C, et al. The ménage à trois of autophagy, lipid droplets and liver disease. Autophagy. 2022;18:50–72.
Fougerat A, Bruse J, Polizzi A, Montagner A, Guillou H, Wahli W. Lipid sensing by PPARα: Role in controlling hepatocyte gene regulatory networks and the metabolic response to fasting. Prog Lipid Res. 2024;96:101303.
Zhang J, Fu M, Cui T, Xiong C, Xu K, Zhong W, et al. Selective disruption of PPARγ2 impairs the development of adipose tissue and insulin sensitivity. Proc Natl Acad Sci USA. 2004;101:10703–8.
Mansoori S, Ho MY, Ng KK, Cheng KK. Branched-chain amino acid metabolism: Pathophysiological mechanism and therapeutic intervention in metabolic diseases. Obes Rev. 2025;26:e13856.
Ning Z, Guo X, Liu X, Lu C, Wang A, Wang X, et al. USP22 regulates lipidome accumulation by stabilizing PPARγ in hepatocellular carcinoma. Nat Commun. 2022;13:2187.
Liu P, Song X, Chen Q, Cen L, Tang C, Yu C, et al. Ubiquitin-specific peptidase 25 ameliorates hepatic steatosis by stabilizing peroxisome proliferator-activated receptor alpha. J Biol Chem. 2024;300:107876.
Zhang DG, Kunz WS, Lei XJ, Zito E, Zhao T, Xu YC, et al. Selenium ameliorated oxidized fish oil-induced lipotoxicity via the inhibition of mitochondrial oxidative stress, remodeling of Usp4-mediated deubiquitination, and stabilization of Pparα. Antioxid Redox Signal. 2024;40:433–52.
Lee KW, Cho JG, Kim CM, Kang AY, Kim M, Ahn BY, et al. Herpesvirus-associated ubiquitin-specific protease (HAUSP) modulates peroxisome proliferator-activated receptor γ (PPARγ) stability through its deubiquitinating activity. J Biol Chem. 2013;288:32886–96.
Jin L, Zhu W, Hu X, Ye L, Lou S, Zhang Q, et al. USP25 directly interacts with and deubiquitinates PPARα to increase PPARα stability in hepatocytes and attenuate high-fat diet-induced MASLD in mice. Cell Death Differ. 2025;32:1112–27.
Dai J, Zhang L, Zhang R, Ge J, Yao F, Zhou S, et al. Hepatocyte deubiquitinating enzyme OTUD5 deficiency is a key aggravator for metabolic dysfunction-associated steatohepatitis by disturbing mitochondrial homeostasis. Cell Mol Gastroenterol Hepatol. 2024;17:399–421.
Hu S, Wang Z, Zhu K, Shi H, Qin F, Zhang T, et al. USP29 alleviates the progression of MASLD by stabilizing ACSL5 through K48 deubiquitination. Clin Mol Hepatol. 2025;31:147–65.
Ishtiaq SM, Arshad MI, Khan JA. PPARγ signaling in hepatocarcinogenesis: Mechanistic insights for cellular reprogramming and therapeutic implications. Pharm Ther. 2022;240:108298.
Wu L, Guo C, Wu J. Therapeutic potential of PPARγ natural agonists in liver diseases. J Cell Mol Med. 2020;24:2736–48.
Yang S, L Xiong, T Liao, L Li, Y Li, L. Kang, et al. Deubiquitinating enzyme USP2 alleviates muscle atrophy by stabilizing PPARγ. Diabetes. 2025;74:773–86.
Luo H, Ji Y, Gao X, Liu X, Wu Y, Wu Y. Ubiquitin specific protease 2: structure, isoforms, cellular function, related diseases and its inhibitors. Oncologie. 2022;24:85–99.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (82400092, 82470152), the Shandong Provincial Natural Science Foundation (ZR2024MH161), the Weifang Science and Technology Development Plan Project (Medical)(2025YX032), the China Postdoctoral Science Foundation (2023M742311, 2024M762019), and the Shanghai Jiao Tong University School of Medicine the 18th Training Program for Innovation and Entrepreneurship (1824010).
Author information
Authors and Affiliations
Contributions
HL and YW contributed to the literature search and study design. HL, YW, PH, and ZZ participated in the drafting of the article. HL, CZ, YW, YD, WB, YZ, YS, YZ, and WW carried out the experiments. YW, MH, YW, ZZ, HL, and HX revised the manuscript. PH, HC, and JW contributed to data collection and analysis. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
The collection and use of human samples were approved by the Ethics Committee of Shandong Second Medical University (Approval Number: SDSMU2024YX297) and strictly adhered to the principles of the Declaration of Helsinki. All participants or their close relatives signed written informed consent forms. All animal experiments were conducted with the approval of the Animal Ethics Committee of Shanghai Jiao Tong University School of Medicine (Approval Number: JUMC2023-027-A). All animal experiments were strictly conducted in accordance with institutional guidelines.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, H., Zhu, C., Wang, Y. et al. USP2 promotes metabolic dysfunction-associated steatotic liver disease progression via stabilization of PPARγ. Cell Death Differ (2025). https://doi.org/10.1038/s41418-025-01589-2
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41418-025-01589-2
This article is cited by
-
Deubiquitinases in liver diseases: from mechanisms to targeted therapy
Science China Life Sciences (2025)