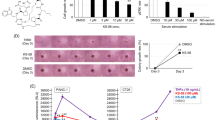
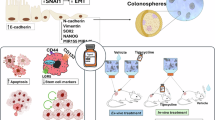
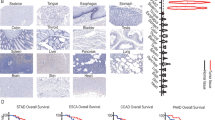
Abstract
ARF GTPase protein 1 (GIT1) is a scaffold protein that is overexpressed in hepatocellular carcinoma (HCC) and colorectal cancer (CRC). GIT1 forms a complex with methionine adenosyltransferase 2B (MAT2B) that activates RAS-RAF-MEK-ERK signaling in HCC and CRC to enhance tumorigenicity. Here, we investigated in a proof-of-concept study whether a small molecule that disrupts GIT1-MAT2B interaction can be effective in HCC and CRC treatment. Since the GIT1 crystal structure is unavailable, we developed a molecular model and used computer-based drug discovery approach to screen for small molecules targeting the GIT1 ankyrin repeat domain, the region closest to where MAT2B interacts that is accessible. Of nine compounds tested, compound 3 (C3) selectively interacts with GIT1 and shows an anti-cancer effect in a GIT1-dependent manner. C3 is antiproliferative, induced apoptosis and G2/M cell cycle arrest while inhibiting colony formation and migration in liver and colon cancer cells. C3 lowered interaction between GIT1 and MAT2B, and with downstream effectors cRAF, MEK and ERK, lowering MEK activity and cyclin D1 expression. Unexpectedly, C3 stabilized GIT1 interaction with cyclin B1 while weakening cyclin B1’s interaction with components of the anaphase promoting complex, concomitant with sustained cyclin B1 expression and mitosis arrest. In mice, C3 administration was well tolerated and inhibited murine CRC growth and liver metastasis in immune competent mice and human CRC growth in the livers of nude mice. In conclusion, a small molecule inhibitor that disrupts GIT1’s normal interactome is a promising new approach to treating liver and colon cancers.
Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this paper and in supplemental information files. All original and uncropped western blots are included in the Supplemental Material.
References
Yang JD, Hainut P, Gores GJ, Amadou A, Plymouth A, Roberts LR. A global review of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604.
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus Bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905.
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Lancet. 2019;394:1467–80.
Zacharakis M, Xynos ID, Lazaris A, Smaro T, Kosmas C, Dokou A, et al. Predictors of survival in stage IV metastatic colorectal cancer. Anticancer Res. 2010;30:653–60.
Peng H, Dara L, Li TWH, Zheng YH, Yang H, Tomasi ML, et al. MAT2B-GIT1 Interplay activates MEK1/ERK 1 and 2 to induce growth in human liver and colon cancer. Hepatology. 2013;57:2299–313.
Peng H, Li TWH, Yang H, Moyer MP, Mato JM, Lu SC. Methionine adenosyltransferase 2B-GIT1 complex serves as a scaffold to regulate Ras/Raf/MEK1/2 activity in human liver and colon cancer cells. Am J Pathol. 2015;185:1135–44.
Yin GY, Haendeler J, Yan C, Berk BC. GIT1 functions as a scaffold for MEK1–extracellular signal-regulated kinase 1 and 2 activation by angiotensin II and epidermal growth factor. Mol Cell Biol. 2004;24:875–85.
Martínez-Chantar ML, Garcia-Trevijano ER, Latasa MU, Martin-Duce A, Fortes P, Caballeria J, et al. Methionine adenosyltransferase II beta subunit gene expression provides a proliferative advantage in human hepatoma. Gastroenterology. 2003;124:940–8.
Yang H, Ara AI, Magilnick N, Xia M, Ramani K, Chen H, et al. Expression pattern, regulation, and functions of methionine adenosyltransferase 2beta splicing variants in hepatoma cells. Gastroenterology. 2008;134:281–91.
Hoefen RJ, Berk BC. The multifunctional GIT1 family of proteins. J Cell Sci. 2006;119:1469–75.
Jerabek-Willemsen M, André T, Wanner R, Roth HM, Duhr S, Baaske P, et al. MicroScale Thermophoresis: Interaction analysis and beyond. J Mol Struct. 2014;1077:101–113.
Munshi A, Hobbs M, Meyn RE. Clonogenic cell survival assay. Methods Mol Med. 2005;110:21–8.
Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog. 2013;18:43–73.
Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–4.
Jin P, Hardy S, Morgan DO. Nuclear localization of cyclin B1 controls mitotic entry after DNA damage. J Cell Biol. 1998;141:875–85.
Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–95.
Santos SDM, Wollman R, Meyer T, Ferrell JrJE. Spatial positive feedback at the onset of mitosis. Cell. 2012;149:1500–13.
Sanchez V, McElroy AK, Spector DH. Mechanisms governing maintenance of Cdk1/Cyclin B1 kinase activity in cells infected with human cytomegalovirus. J Virol. 2003;77:13214.
Sivakumar S, Gorbsky GJ. Spatiotemporal regulation of the anaphase-promoting complex in mitosis. Nat Rev Mol Cell Bio. 2015;16:82–94.
Schrock MS, Stromberg BR, Scarberry L, Summers MK. APC/C ubiquitin ligase: functions and mechanisms in tumorigenesis. Semin Cancer Biol. 2020;67:80–91.
Yamaguchi M, Yu S, Qiao R, Weissmann F, Miller DJ, VanderLinden R, et al. Structure of an APC3-APC16 complex: insights into assembly of the anaphase-promoting complex/cyclosome. J Mol Biol. 2015;427:1748–64.
Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–57.
Lavanya V, Mohamed AAA, Neesar A, Rishi AK, Jamal S. Small molecule inhibitors as emerging cancer therapeutics. Integr Cancer Sci Ther. 2014;1:39–46.
Bollag G, Tsai J, Zhang J, Zhang C, Ibrahim P, Nolop K, et al. Vemurafenib: the first drug approved for BRAF-mutant cancer. Nat Rev Drug Discov. 2012;1:873–86.
Roskoski R. RAF protein-serine/threonine kinases: structure and regulation. Biochem Biophys Res Commun. 2010;399:313–7.
Roskoski R. ERK1/2 MAP kinases: structure, function, and regulation. Pharm Res. 2012;66:105–43.
Hinoue T, Weisenberger DJ, Pan F, Campan M, Kim M, Young J, et al. Analysis of the association between CIMP and BRAFV600E in colorectal cancer by DNA methylation profiling. PLoS One. 2009;4:e8357.
Narayan S, Roy D. Role of APC and DNA mismatch repair genes in the development of colorectal cancers. Mol Cancer. 2003;2:41.
Ahmed O, Eide PW, Eilertsen IA, Danielsen SA, Eknæs M, Hektoen M, et al. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis. 2013;2:e71.
Scherer D, López MD, Goeppert B, Abrahamsson S, Silos RG, Nova I, et al. RNA Sequencing Of Hepatobiliary Cancer Cell Lines: Data And Applications To Mutational And Transcriptomic Profiling. Cancers. 2020;12:2510.
Gavet O, Pines J. Activation of cyclin B1–Cdk1 synchronizes events in the nucleus and the cytoplasm at mitosis. J Cell Biol. 2010;189:247–59.
Hain KO, Colin DJ, Rastogi S, Allan LA, Clarke PR. Prolonged mitotic arrest induces a caspase-dependent DNA damage response at telomeres that determines cell survival. Sci Rep. 2016;6:26766.
Egorshina AY, Zamaraev AV, Kaminskyy VO, Radygina TV, Zhivotovsky B, Kopeina GS. Necroptosis as a novel facet of mitotic catastrophe. Int J Mol Sci. 2022;23:3733.
Pang J, Yan C, Natarajan K, Cavet ME, Massett MP, Yin G, et al. GIT1 mediates HDAC5 activation by angiotensin II in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28:892–8.
Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol Cell. 2005;20:237–49.
Nigg EA, Stearns T. The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries. Nat Cell Biol. 2011;13:1154–60.
Yang HP, Li TWH, Ko KS, Xia M, Lu SC. Switch from Mnt-Max to Myc-Max induces p53 and cyclin D1 expression and apoptosis during cholestasis in mice and human hepatocytes. Hepatology. 2009;49:860–70.
Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008;9:40.
Detre S, Jotti GS, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48:876–8.
Acknowledgements
Authors acknowledge Tony TW Li’s contribution in the initial screening of the GIT1 inhibitors. This work was supported by NIH grant P01CA233452 (SC Lu, ML Tomasi), Plan Nacional of I + D SAF2017-88041-R (JM Mato). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
HP, JC, WF, JW, AC, RM, YZ – conducted the experiments, data collection, drafted methods; JC, LBT, RM – prepared figures; LBT, SS, MLT, RM, JMM – critical reading of the manuscript; SCL - study concept and design, data analysis and interpretation, edited/wrote the manuscript, obtained funding, and provided overall study supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
SCL and RM have filed a patent for small molecule inhibitors of GIT1 (Title: COMPOUNDS AND METHODS FOR TREATING CANCERS; Application No.: 63/422,672; Filed: November 4, 2022). The other authors declare that they have no competing interests.
Ethics statement
We confirm that all experiments were performed in accordance with relevant guidelines and regulations and approved by the Institutional Animal Care and Use Committee of Cedars-Sinai Medical Center.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Quan Chen
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peng, H., Chhimwal, J., Fan, W. et al. A small molecule inhibitor of ARF GTPase protein 1 limits liver and colon cancer cell growth and metastasis. Cell Death Dis (2026). https://doi.org/10.1038/s41419-026-08477-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-026-08477-8