Abstract
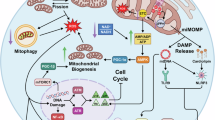
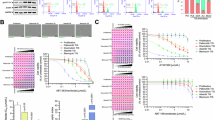
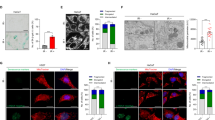
Mitochondria integrate senescence and apoptotic fates, yet it is unclear whether their ability to oxidize different fuels for energy production influences their vulnerability to senolytics in therapy-induced senescence (TIS). Using MitoPlates™ technology, we functionally mapped the mitophenotypes of TIS cancer cells by quantifying electron transport chain (ETC) flux from various NADH/FADH2 substrates. We then related these profiles to the responsiveness of TIS cancer cells to BCL-xL-targeting BH3 senolytics, as well as to inflammatory SASP signaling sensed by an NF-κB/miR-146a reporter. Mechanistically distinct senogenic stressors produced markedly different bioenergetic outputs and substrate diversity, establishing mitochondria as an emergent, stress-encoded property of TIS phenomena. Increased mitochondrial bioenergetic flexibility corresponded with senolytic permissiveness within each cell lineage. However, the magnitude of the senolytic response was largely limited by the pre-senescent bioenergetic configuration of the parental mitochondria, and baseline succinate oxidation served as a functional indicator of this inherited threshold. TIS SASPs were restricted by the secretome of the cell-of-origin, but only the miR146a-positive, fatty acid β-oxidation-related inflammatory SASP states were senolytically responsive. Inflachromene, an inhibitor of the chromatin remodelers HMGB1/2, decoupled mitochondrial bioenergetics from senolytic susceptibility, yielding SASP-null/miR146a-negative senescent cancer cells that were completely resistant to ABT-263/navitoclax and A1331852 despite extensive mitochondrial reprogramming. Thus, the senolytic response is governed by a layered circuit in which mitochondrial bioenergetic heritage establishes the senolytic ceiling, TIS-acquired bioenergetic flexibility fine-tunes the amplitude of the senolytic response, and establishing a mitochondria-inflammatory SASP crosstalk is required for BH3-mediated senolysis. These results support using functional readouts that integrate mitochondrial metabolic flexibility and inflammatory SASP to predict and potentially enhance senolytic efficacy in TIS cancer cells.
Similar content being viewed by others
Data availability
All of the data sets used in the present study are available from the corresponding authors upon reasonable request.
References
Monzel AS, Enríquez JA, Picard M. Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction. Nat Metab. 2023;5:546–62.
Gallage S, Gil J. Mitochondrial dysfunction meets senescence. Trends Biochem Sci. 2016;41:207–9.
Miwa S, Kashyap S, Chini E, von Zglinicki T. Mitochondrial dysfunction in cell senescence and aging. J Clin Investig. 2022;132:e158447.
Martini H, Passos JF. Cellular senescence: all roads lead to mitochondria. FEBS J. 2023;290:1186–202.
Correia-Melo C, Passos JF. Mitochondria: are they causal players in cellular senescence? Biochim Biophys Acta. 2015;1847:1373–9.
Correia-Melo C, Marques FD, Anderson R, Hewitt G, Hewitt R, Cole J, et al. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J. 2016;35:724–42.
Victorelli S, Salmonowicz H, Chapman J, Martini H, Vizioli MG, Riley JS, et al. Apoptotic stress causes mtDNA release during senescence and drives the SASP. Nature. 2023;622:627–36.
Moiseeva O, Bourdeau V, Roux A, Deschênes-Simard X, Ferbeyre G. Mitochondrial dysfunction contributes to oncogene-induced senescence. Mol Cell Biol. 2009;29:4495–507.
Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, et al. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab. 2016;23:303–14.
Vizioli MG, Liu T, Miller KN, Robertson NA, Gilroy K, Lagnado AB, et al. Mitochondria-to-nucleus retrograde signaling drives formation of cytoplasmic chromatin and inflammation in senescence. Genes Dev. 2020;34:428–45.
Takebayashi S, Tanaka H, Hino S, Nakatsu Y, Igata T, Sakamoto A, et al. Retinoblastoma protein promotes oxidative phosphorylation through upregulation of glycolytic genes in oncogene-induced senescent cells. Aging Cell. 2015;14:689–97.
Quijano C, Cao L, Fergusson MM, Romero H, Liu J, Gutkind S, et al. Oncogene-induced senescence results in marked metabolic and bioenergetic alterations. Cell Cycle. 2012;11:1383–92.
Wang L, Bernards R. Taking advantage of drug resistance, a new approach in the war on cancer. Front Med. 2018;12:490–5.
Prasanna PG, Citrin DE, Hildesheim J, Ahmed MM, Venkatachalam S, Riscuta G, et al. Therapy-induced senescence: opportunities to improve anticancer therapy. J Natl Cancer Inst. 2021;113:1285–98.
Wang L, Lankhorst L, Bernards R. Exploiting senescence for the treatment of cancer. Nat Rev Cancer. 2022;22:340–55.
Schmitt CA, Wang B, Demaria M. Senescence and cancer—role and therapeutic opportunities. Nat Rev Clin Oncol. 2022;19:619–36.
Settleman J, Neto JMF, Bernards R. Thinking differently about cancer treatment regimens. Cancer Discov. 2021;11:1016–23.
Gonzalez LC, Ghadaouia S, Martinez A, Rodier F. Premature aging/senescence in cancer cells facing therapy: good or bad? Biogerontology. 2016;17:71–87.
Saleh T, Bloukh S, Carpenter VJ, Alwohoush E, Bakeer J, Darwish S, et al. Therapy-induced senescence: an “Old” friend becomes the enemy. Cancers. 2020;12:822.
Wang B, Kohli J, Demaria M. Senescent cells in cancer therapy: friends or foes? Trends Cancer. 2020;6:838–57.
Marin I, Boix O, García-Garijo A, Sirois I, Caballe A, Zarzuela E, et al. Cellular senescence is immunogenic and promotes antitumor immunity. Cancer Discov. 2023;13:410–31.
Chen HA, Ho YJ, Mezzadra R, Adrover JM, Smolkin R, Zhu C, et al. Senescence rewires microenvironment sensing to facilitate antitumor immunity. Cancer Discov. 2023;13:432–53.
Saleh T, Tyutyunyk-Massey L, Gewirtz DA. Tumor cell escape from therapy-induced senescence as a model of disease recurrence after dormancy. Cancer Res. 2019;79:1044–6.
Faheem MM, Seligson ND, Ahmad SM, Rasool RU, Gandhi SG, Bhagat M, et al. Convergence of therapy-induced senescence (TIS) and EMT in multistep carcinogenesis: current opinions and emerging perspectives. Cell Death Discov. 2020;6:51.
Santos-de-Frutos K, Djouder N. When dormancy fuels tumour relapse. Commun Biol. 2021;4:747.
Chibaya L, Snyder J, Ruscetti M. Senescence and the tumor-immune landscape: Implications for cancer immunotherapy. Semin Cancer Biol. 2022;86:827–45.
Demaria M, O’Leary MN, Chang J, Shao L, Liu S, Alimirah F, et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017;7:165–76.
Guida JL, Agurs-Collins T, Ahles TA, Campisi J, Dale W, Demark-Wahnefried W, et al. Strategies to prevent or remediate cancer and treatment-related aging. J Natl Cancer Inst. 2021;113:112–22.
Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM, et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169:132–47.
Fielder E, Wan T, Alimohammadiha G, Ishaq A, Low E, Weigand BM, et al. Short senolytic or senostatic interventions rescue progression of radiation-induced frailty and premature ageing in mice. Elife. 2022;11:e75492.
Wang L, Leite de Oliveira R, Wang C, Fernandes Neto JM, Mainardi S, Evers B, et al. High-throughput functional genetic and compound screens identify targets for senescence induction in cancer. Cell Rep. 2017;21:773–83.
Wang C, Vegna S, Jin H, Benedict B, Lieftink C, Ramirez C, et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature. 2019;574:268–72.
Zhang JW, Zhang D, Yu BP. Senescent cells in cancer therapy: why and how to remove them. Cancer Lett. 2021;520:68–79.
L’Hôte V, Mann C, Thuret JY. From the divergence of senescent cell fates to mechanisms and selectivity of senolytic drugs. Open Biol. 2022;12:220171.
Basu A. The interplay between apoptosis and cellular senescence: Bcl-2 family proteins as targets for cancer therapy. Pharmacol Ther. 2022;230:107943.
Hinz B, Lagares D. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat Rev Rheumatol. 2020;16:11–31.
Merkt W, Bueno M, Mora AL, Lagares D. Senotherapeutics: targeting senescence in idiopathic pulmonary fibrosis. Semin Cell Dev Biol. 2020;101:104–10.
Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun. 2016;7:11190.
Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83.
Shahbandi A, Rao SG, Anderson AY, Frey WD, Olayiwola JO, Ungerleider NA, et al. BH3 mimetics selectively eliminate chemotherapy-induced senescent cells and improve response in TP53 wild-type breast cancer. Cell Death Differ. 2020;27:3097–116.
Jochems F, Thijssen B, De Conti G, Jansen R, Pogacar Z, Groot K, et al. The cancer SENESCopedia: a delineation of cancer cell senescence. Cell Rep. 2021;36:109441.
Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15:428–35.
Zhu Y, Doornebal EJ, Pirtskhalava T, Giorgadze N, Wentworth M, Fuhrmann-Stroissnigg H, et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging. 2017;9:955–63.
Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–84.
Kohli J, Ge C, Fitsiou E, Doepner M, Brandenburg SM, Faller WJ, et al. Targeting anti-apoptotic pathways eliminates senescent melanocytes and leads to nevi regression. Nat Commun. 2022;13:7923.
Saleh T, Carpenter VJ, Tyutyunyk-Massey L, Murray G, Leverson JD, Souers AJ, et al. Clearance of therapy-induced senescent tumor cells by the senolytic ABT-263 via interference with BCL-XL -BAX interaction. Mol Oncol. 2020;14:2504–19.
Malaquin N, Vancayseele A, Gilbert S, Antenor-Habazac L, Olivier MA, Ait Ali Brahem Z, et al. DNA damage- but not enzalutamide-induced senescence in prostate cancer promotes senolytic Bcl-xL inhibitor sensitivity. Cells. 2020;9:1593.
MacDonald JA, Bradshaw GA, Jochems F, Bernards R, Letai A. Apoptotic priming in senescence predicts specific senolysis by quantitative analysis of mitochondrial dependencies. Cell Death Differ. 2025;32:802–17.
Jochems F, Baltira C, MacDonald JA, Daniels V, Mathur A, de Gooijer MC, et al. Senolysis by ABT-263 is associated with inherent apoptotic dependence of cancer cells derived from the non-senescent state. Cell Death Differ. 2025;32:855–65.
Alcon C, Kovatcheva M, Morales-Sánchez P, López-Polo V, Torres T, Puig S, et al. HRK downregulation and augmented BCL-xL binding to BAK confer apoptotic protection to therapy-induced senescent melanoma cells. Cell Death Differ. 2025;32:646–56.
López J, Llop-Hernández À, Verdura S, Serrano-Hervás E, Martínez-Balibrea E, Bosch-Barrera J, et al. Mitochondrial priming and response to BH3 mimetics in “one-two punch” senogenic-senolytic strategies. Cell Death Discov. 2025;11:91.
Menendez JA, Lupu R, Martin-Castillo B, Sardanyés J, Alarcón T, Verdura S, et al. Mitochondrial priming in therapy-induced senescence: implications for CAR-T/NK immunosenolytic therapy. Front Immunol. 2025;16:1695244.
Radogna F, Gérard D, Dicato M, Diederich M. Assessment of mitochondrial cell metabolism by respiratory chain electron flow assays. Methods Mol Biol. 2021;2276:129–41.
Kordus RJ, Hossain A, Malter HE, LaVoie HA. Mitochondrial metabolic substrate utilization in granulosa cells reflects body mass index and total follicle-stimulating hormone dosage in in vitro fertilization patients. J Assist Reprod Genet. 2020;37:2743–56.
Tramonti A, Cuyàs E, Encinar JA, Pietzke M, Paone A, Verdura S, et al. Metformin is a pyridoxal-5’-phosphate (PLP)-competitive inhibitor of SHMT2. Cancers. 2021;13:4009.
Cuyàs E, Fernández-Arroyo S, Verdura S, Lupu R, Joven J, Menéndez JA. Metabolomic and mitochondrial fingerprinting of the epithelial-to-mesenchymal transition (EMT) in non-tumorigenic and tumorigenic human breast cells. Cancers. 2022;14:6214.
Carreira ASA, Ravera S, Zucal C, Thongon N, Caffa I, Astigiano C, et al. Mitochondrial rewiring drives metabolic adaptation to NAD(H) shortage in triple-negative breast cancer cells. Neoplasia. 2023;41:100903.
Kang C, Xu Q, Martin TD, Li MZ, Demaria M, Aron L, et al. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science. 2015;349:aaa5612.
Bhaumik D, Scott GK, Schokrpur S, Patil CK, Orjalo AV, Rodier F, et al. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging. 2009;1:402–11.
Palikyras S, Sofiadis K, Stavropoulou A, Danieli-Mackay A, Varamogianni-Mamatsi V, Hörl D, et al. Rapid and synchronous chemical induction of replicative-like senescence via a small molecule inhibitor. Aging Cell. 2024;23:e14083.
Aird KM, Iwasaki O, Kossenkov AV, Tanizawa H, Fatkhutdinov N, Bitler BG, et al. HMGB2 orchestrates the chromatin landscape of senescence-associated secretory phenotype gene loci. J Cell Biol. 2016;215:325–34.
Guerrero A, Gil J. HMGB2 holds the key to the senescence-associated secretory phenotype. J Cell Biol. 2016;215:297–9.
Sofiadis K, Josipovic N, Nikolic M, Kargapolova Y, Übelmesser N, Varamogianni-Mamatsi V, et al. HMGB1 coordinates SASP-related chromatin folding and RNA homeostasis on the path to senescence. Mol Syst Biol. 2021;17:e9760.
Wiley CD, Campisi J. From ancient pathways to aging cells-connecting metabolism and cellular senescence. Cell Metab. 2016;23:1013–21.
Tesei A, Arienti C, Bossi G, Santi S, De Santis I, Bevilacqua A, et al. TP53 drives abscopal effect by secretion of senescence-associated molecular signals in non-small cell lung cancer. J Exp Clin Cancer Res. 2021;40:89.
Aoshiba K, Tsuji T, Nagai A. Bleomycin induces cellular senescence in alveolar epithelial cells. Eur Respir J. 2003;22:436–43.
Tsuji T, Aoshiba K, Nagai A. Cigarette smoke induces senescence in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2004;31:643–9.
Vilgelm AE, Johnson CA, Prasad N, Yang J, Chen SC, Ayers GD, et al. Connecting the dots: therapy-induced senescence and a tumor-suppressive immune microenvironment. J Natl Cancer Inst. 2015;108:djv406.
Saleh T, Tyutyunyk-Massey L, Murray GF, Alotaibi MR, Kawale AS, Elsayed Z, et al. Tumor cell escape from therapy-induced senescence. Biochem Pharmacol. 2019;162:202–12.
Karabicici M, Alptekin S, Fırtına Karagonlar Z, Erdal E. Doxorubicin-induced senescence promotes stemness and tumorigenicity in EpCAM-/CD133- nonstem cell population in hepatocellular carcinoma cell line, HuH-7. Mol Oncol. 2021;15:2185–202.
Wagner V, Gil J. Senescence as a therapeutically relevant response to CDK4/6 inhibitors. Oncogene. 2020;39:5165–76.
Llanos S, Megías D, Blanco-Aparicio C, Hernández-Encinas E, Rovira M, Pietrocola F, et al. Lysosomal trapping of palbociclib and its functional implications. Oncogene. 2019;38:3886–902.
Wang B, Varela-Eirin M, Brandenburg SM, Hernandez-Segura A, van Vliet T, Jongbloed EM, et al. Pharmacological CDK4/6 inhibition reveals a p53-dependent senescent state with restricted toxicity. EMBO J. 2022;41:e108946.
Crozier L, Foy R, Mouery BL, Whitaker RH, Corno A, Spanos C, et al. CDK4/6 inhibitors induce replication stress to cause long-term cell cycle withdrawal. EMBO J. 2022;41:e108599.
Lee DH, Imran M, Choi JH, Park YJ, Kim YH, Min S, et al. CDK4/6 inhibitors induce breast cancer senescence with enhanced anti-tumor immunogenic properties compared with DNA-damaging agents. Mol Oncol. 2024;18:216–32.
Nehme J, Maassen S, Bravaccini S, Zanoni M, Gianni C, De Giorgi U, et al. Pharmacological CDK4/6 inhibition promotes vulnerability to lysosomotropic agents in breast cancer. EMBO J. 2025;44:1921–42.
Dragulska S, Kańska M. Enzymatic synthesis of tryptamine and its halogen derivatives selectively labeled with hydrogen isotopes. J Radioanal Nucl Chem. 2014;299:759–63.
Sullivan JP, McDonnell L, Hardiman OM, Farrell MA, Phillips JP, Tipton KF. The oxidation of tryptamine by the two forms of monoamine oxidase in human tissues. Biochem Pharmacol. 1986;35:3255–60.
Wang CC, Billett E, Borchert A, Kuhn H, Ufer C. Monoamine oxidases in development. Cell Mol Life Sci. 2013;70:599–630.
Lei XH, Bochner BR. Optimization of cell permeabilization in electron flow-based mitochondrial function assays. Free Radic Biol Med. 2021;177:48–57.
Izzo LT, Trefely S, Demetriadou C, Drummond JM, Mizukami T, Kuprasertkul N, et al. Acetylcarnitine shuttling links mitochondrial metabolism to histone acetylation and lipogenesis. Sci Adv. 2023;9:eadf0115.
Yamauchi S, Sugiura Y, Yamaguchi J, Zhou X, Takenaka S, Odawara T, et al. Mitochondrial fatty acid oxidation drives senescence. Sci Adv. 2024;10:eado5887.
Martini H, Birch J, Marques F, Victorelli S, Lagnado A, Pirius N, et al. Mitochondrial metabolism and epigenetic crosstalk drive the SASP. Res Sq. 2024. Preprint at https://doi.org/10.21203/rs3.rs-5278203/v1.
López-Polo V, Maus M, Zacharioudakis E, Lafarga M, Attolini CS, Marques FDM, et al. Release of mitochondrial dsRNA into the cytosol is a key driver of the inflammatory phenotype of senescent cells. Nat Commun. 2024;15:7378.
Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–68.
Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118.
Ortiz-Montero P, Londoño-Vallejo A, Vernot JP. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu, strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun Signal. 2017;15:17.
Tripathi U, Suda M, Kulshreshtha V, Piatkowski BT, Palmer AK, Giorgadze N, et al. Senolytic-resistant senescent cells have a distinct sasp profile and functional impact: the path to developing senosensitizers. Aging Cell. 2026;25:e70358.
Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–6.
Lukiw WJ, Zhao Y, Cui JG. An NF-kappaB-sensitive microRNA-146a-mediated inflammatory circuit in Alzheimer's disease and in stressed human brain cells. J Biol Chem. 2008;283:31315–22.
Saba R, Sorensen DL, Booth SA. MicroRNA-146a: a dominant, negative regulator of the innate immune response. Front Immunol. 2014;5:578.
Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023.
Guo Q, Jin Y, Chen X, Ye X, Shen X, Lin M, et al. NF-κB in biology and targeted therapy: new insights and translational implications. Signal Transduct Target Ther. 2024;9:53.
Mao H, Zhao X, Sun SC. NF-κB in inflammation and cancer. Cell Mol Immunol. 2025;22:811–39.
Altea-Manzano P, Doglioni G, Liu Y, Cuadros AM, Nolan E, Fernández-García J, et al. A palmitate-rich metastatic niche enables metastasis growth via p65 acetylation, resulting in pro-metastatic NF-κB signaling. Nat Cancer. 2023;4:344–64.
Bousset L, Gil J. Targeting senescence as an anticancer therapy. Mol Oncol. 2022;16:3855–80.
Maggiorani D, Manzella N, Edmondson DE, Mattevi A, Parini A, Binda C, et al. Monoamine oxidases, oxidative stress, and altered mitochondrial dynamics in cardiac ageing. Oxid Med Cell Longev. 2017;2017:3017947.
Manzella N, Santin Y, Maggiorani D, Martini H, Douin-Echinard V, Passos JF, et al. Monoamine oxidase-A is a novel driver of stress-induced premature senescence through inhibition of parkin-mediated mitophagy. Aging Cell. 2018;17:e12811.
Santin Y, Resta J, Parini A, Mialet-Perez J. Monoamine oxidases in age-associated diseases: new perspectives for old enzymes. Ageing Res Rev. 2021;66:101256.
Santin Y, Fazal L, Sainte-Marie Y, Sicard P, Maggiorani D, Tortosa F, et al. Mitochondrial 4-HNE derived from MAO-A promotes mitoCa2+ overload in chronic postischemic cardiac remodeling. Cell Death Differ. 2020;27:1907–23.
Mialet-Perez J, Parini A. Cardiac monoamine oxidases: at the heart of mitochondrial dysfunction. Cell Death Dis. 2020;11:54.
Kugapreethan R, Elahee Doomun SN, Sacharz J, Frazier AE, Sharma T, Low YC, et al. Complex II assembly drives metabolic adaptation to OXPHOS dysfunction. Sci Adv. 2025;11:eadr6012.
Zhang L, Pitcher LE, Prahalad V, Niedernhofer LJ, Robbins PD. Targeting cellular senescence with senotherapeutics: senolytics and senomorphics. FEBS J. 2023;290:1362–83.
Feng T, Xie F, Lee LMY, Lin Z, Tu Y, Lyu Y, et al. Cellular senescence in cancer: from mechanism paradoxes to precision therapeutics. Mol Cancer. 2025;24:213.
McHugh D, Durán I, Gil J. Senescence as a therapeutic target in cancer and age-related diseases. Nat Rev Drug Discov. 2025;24:57–71.
Chapman J, Fielder E, Passos JF. Mitochondrial dysfunction and cell senescence: deciphering a complex relationship. FEBS Lett. 2019;593:1566–79.
Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–96.
Acknowledgements
Schematics in Figs. 1, 2 and 7 were created with BioRender.com.
Funding
The work in the Javier A. Menendez laboratory is supported by the Ministerio de Ciencia e Innovación and the Spanish Research Agency (MCIN/AEI, grant PID2022-141955OB-I00, Plan Nacional de I+D+i, funded by MCIN/AEI/10.13039/501100011033/FEDER/UE "ERDF A way of making Europe" funded by the European Regional Development Fund), and the Emerging Research Group SGR 2021 01507 of the Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR, Generalitat de Catalunya) to Begoña Martin-Castillo. Elisabet Cuyàs holds a “Miguel Servet” research contract (CP20/00003) from the Instituto de Salud Carlos III (Spain) and is supported by the grant PI22/00297 (Instituto de Salud Carlos III, Proyectos de I + D + I en Salud, Acción Estratégica en Salud 2021–2023, funded by the “ERDF A way of making Europe”). Elisabet Cuyàs and Javier A. Menendez thank the CERCA Program/Generalitat de Catalunya for the institutional support of IDIBGI.
Author information
Authors and Affiliations
Contributions
AL-H: Conceptualization, Methodology, Investigation, Validation, Data Curation, Formal analysis, Visualization. SV: Conceptualization, Methodology, Investigation, Data Curation, Formal analysis. Visualization. JL: Methodology, Investigation, Validation, Data Curation, Formal analysis, Visualization. BM-C: Project administration, Methodology, Investigation. JAM: Conceptualization, Project administration, Resources, Supervision, Methodology, Visualization, Writing–original draft, Writing—review and editing. EC: Conceptualization, Project administration, Supervision, Resources, Methodology, Formal analysis, Visualization, Writing–original draft, Writing—review and editing. All authors are in agreement on the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All authors agreed to the publication of the article. All data were in-house, and no paper mills were used. To ensure integrity and accuracy, all authors agree to take responsibility for all aspects of the work. The authors did not use any AI tool/service or AI-enabled technologies in the preparation of this paper.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. None of the experiments in this study involved the use of materials (i.e., human or mouse samples) that are subject to ethical approval.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Llop-Hernández, À., Verdura, S., López, J. et al. Mitochondrial bioenergetics-SASP crosstalk determines senolytic efficacy in therapy-induced senescence. Cell Death Discov. (2026). https://doi.org/10.1038/s41420-026-02967-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-026-02967-6