Abstract
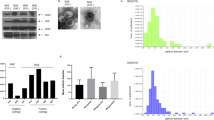
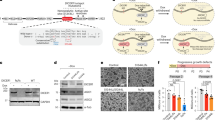
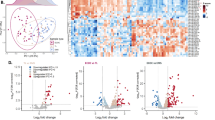
Reduced expression of DICER, a key enzyme in the miRNA pathway, is frequently associated with aggressive, invasive disease, and poor survival in various malignancies. Regulation of DICER expression is, however, poorly understood. Here, we show that NF90/NF110 facilitates DICER expression by controlling the processing of a miRNA, miR-3173, which is embedded in DICER pre-mRNA. As miR-3173 in turn targets NF90, a feedback amplification loop controlling DICER expression is established. In a nude mouse model, NF90 overexpression reduced proliferation of ovarian cancer cells and significantly reduced tumor size and metastasis, whereas overexpression of miR-3173 dramatically increased metastasis in an NF90- and DICER-dependent manner. Clinically, low NF90 expression and high miR-3173-3p expression were found to be independent prognostic markers of poor survival in a cohort of ovarian carcinoma patients. These findings suggest that, by facilitating DICER expression, NF90 can act as a suppressor of ovarian carcinoma.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386 (2015).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 66, 7–30 (2016).
Bast, R. C. Jr., Hennessy, B. & Mills, G. B. The biology of ovarian cancer: new opportunities for translation. Nat. Rev. Cancer 9, 415–428 (2009).
Jemal, A. et al. Cancer statistics, 2004. CA Cancer J. Clin. 54, 8–29 (2004).
Naora, H. & Montell, D. J. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat. Rev. Cancer 5, 355–366 (2005).
Buys, S. S. et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 305, 2295–2303 (2011).
Mei, L. et al. Maintenance chemotherapy for ovarian cancer. Cochrane Database Syst. Rev. CD007414 (2013).
Denli, A. M., Tops, B. B., Plasterk, R. H., Ketting, R. F. & Hannon, G. J. Processing of primary microRNAs by the Microprocessor complex. Nature 432, 231–235 (2004).
Gregory, R. I. et al. The microprocessor complex mediates the genesis of microRNAs. Nature 432, 235–240 (2004).
Han, J. et al. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 18, 3016–3027 (2004).
Lee, Y. et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 425, 415–419 (2003).
Bernstein, E., Caudy, A. A., Hammond, S. M. & Hannon, G. J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409, 363–366 (2001).
Grishok, A. et al. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 106, 23–34 (2001).
Ketting, R. F. et al. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 15, 2654–2659 (2001).
Knight, S. W. & Bass, B. L. A role for the RNase III enzyme DCR-1 in RNA interference and germ line development in Caenorhabditis elegans. Science 293, 2269–2271 (2001).
Ha, M. & Kim, V. N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 15, 509–524 (2014).
Lin, S. & Gregory, R. I. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer 15, 321–333 (2015).
Lu, J. et al. MicroRNA expression profiles classify human cancers. Nature 435, 834–838 (2005).
Kumar, M. S., Lu, J., Mercer, K. L., Golub, T. R. & Jacks, T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat. Genet. 39, 673–677 (2007).
Kumar, M. S. et al. Dicer1 functions as a haploinsufficient tumor suppressor. Genes Dev. 23, 2700–2704 (2009).
Martello, G. et al. A MicroRNA targeting dicer for metastasis control. Cell 141, 1195–1207 (2010).
Melo, S. A. et al. A genetic defect in exportin-5 traps precursor microRNAs in the nucleus of cancer cells. Cancer Cell 18, 303–315 (2010).
Su, X. et al. TAp63 suppresses metastasis through coordinate regulation of Dicer and miRNAs. Nature 467, 986–990 (2010).
Grelier, G. et al. Prognostic value of Dicer expression in human breast cancers and association with the mesenchymal phenotype. Br. J. Cancer 101, 673–683 (2009).
Karube, Y. et al. Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci. 96, 111–115 (2005).
Merritt, W. M. et al. Dicer, Drosha, and outcomes in patients with ovarian cancer. N. Engl. J. Med. 359, 2641–2650 (2008).
Shan, W. et al. Role of Dicer as a prognostic predictor for survival in cancer patients: a systematic review with a meta-analysis. Oncotarget 7, 72672–72684 (2016).
Jafarnejad, S. M., Ardekani, G. S., Ghaffari, M., Martinka, M. & Li, G. Sox4-mediated Dicer expression is critical for suppression of melanoma cell invasion. Oncogene 32, 2131–2139 (2013).
Levy, C. et al. Lineage-specific transcriptional regulation of DICER by MITF in melanocytes. Cell 141, 994–1005 (2010).
Yu, Z. et al. Cyclin D1 induction of Dicer governs microRNA processing and expression in breast cancer. Nat. Commun. 4, 2812 (2013).
Feinberg-Gorenshtein, G. et al. MiR-192 directly binds and regulates Dicer1 expression in neuroblastoma. PLoS One 8, e78713 (2013).
Forman, J. J., Legesse-Miller, A. & Coller, H. A. A search for conserved sequences in coding regions reveals that the let-7 microRNA targets Dicer within its coding sequence. Proc. Natl. Acad. Sci. USA 105, 14879–14884 (2008).
van den Beucken, T. et al. Hypoxia promotes stem cell phenotypes and poor prognosis through epigenetic regulation of DICER. Nat. Commun. 5, 5203 (2014).
Castella, S., Bernard, R., Corno, M., Fradin, A. & Larcher, J. C. Ilf3 and NF90 functions in RNA biology. Wiley Interdiscip. Rev. RNA 6, 243–256 (2014).
Sakamoto, S. et al. The NF90-NF45 complex functions as a negative regulator in the microRNA processing pathway. Mol. Cell. Biol. 29, 3754–3769 (2009).
Todaka, H. et al. Overexpression of NF90-NF45 represses myogenic microRNA biogenesis, resulting in development of skeletal muscle atrophy and centronuclear muscle fibers. Mol. Cell. Biol. 35, 2295–2308 (2015).
Wagschal, A. et al. Microprocessor, Setx, Xrn2, and Rrp6 co-operate to induce premature termination of transcription by RNAPII. Cell 150, 1147–1157 (2012).
Kim, Y. K. & Kim, V. N. Processing of intronic microRNAs. EMBO J. 26, 775–783 (2007).
Morlando, M. et al. Primary microRNA transcripts are processed co-transcriptionally. Nat. Struct. Mol. Biol. 15, 902–909 (2008).
Wu, C. L. et al. Senescence-associated long non-coding RNA (SALNR) delays oncogene-induced senescence through NF90 regulation. J. Biol. Chem. 290, 30175–30192 (2015).
Corthesy, B. & Kao, P. N. Purification by DNA affinity chromatography of two polypeptides that contact the NF-AT DNA binding site in the interleukin 2 promoter. J. Biol. Chem. 269, 20682–20690 (1994).
Lee, D., Nam, J. W. & Shin, C. DROSHA targets its own transcript to modulate alternative splicing. Rna 23, 1035–1047 (2017).
Wong, N. & Wang, X. miRDB: an online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 43, D146–D152 (2015).
Camp, R. L., Dolled-Filhart, M. & Rimm, D. L. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 10, 7252–7259 (2004).
Pawlicki, J. M. & Steitz, J. A. Subnuclear compartmentalization of transiently expressed polyadenylated pri-microRNAs: processing at transcription sites or accumulation in SC35 foci. Cell Cycle 8, 345–356 (2009).
Schwarzenbach, H., Nishida, N., Calin, G. A. & Pantel, K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat. Rev. Clin. Oncol. 11, 145–156 (2014).
Shabman, R. S. et al. DRBP76 associates with Ebola virus VP35 and suppresses viral polymerase function. J. Infect. Dis. 204(Suppl 3), S911–S918 (2011).
Sobhian, B. et al. HIV-1 Tat assembles a multifunctional transcription elongation complex and stably associates with the 7SK snRNP. Mol. Cell 38, 439–451 (2010).
Li, X. D. et al. Overexpression of maelstrom promotes bladder urothelial carcinoma cell aggressiveness by epigenetically downregulating MTSS1 through DNMT3B. Oncogene 35, 6281–6292 (2016).
Zhang, J. X. et al. Prognostic and predictive value of a microRNA signature in stage II colon cancer: a microRNA expression analysis. Lancet Oncol. 14, 1295–1306 (2013).
Zhang, J. X. et al. Downregulation of microRNA-644a promotes esophageal squamous cell carcinoma aggressiveness and stem cell-like phenotype via dysregulation of PITX2. Clin. Cancer Res. 23, 298–310 (2017).
Bittencourt, D. et al. Cotranscriptional splicing potentiates the mRNA production from a subset of estradiol-stimulated genes. Mol. Cell. Biol. 28, 5811–5824 (2008).
Ran, F. A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Montague, T. G., Cruz, J. M., Gagnon, J. A., Church, G. M. & Valen, E. CHOPCHOP: a CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 42, W401–W407 (2014).
Xie, D. et al. Heterogeneous expression and association of beta-catenin, p16 and c-myc in multistage colorectal tumorigenesis and progression detected by tissue microarray. Int. J. Cancer 107, 896–902 (2003).
Zheng, F. et al. Systemic delivery of microRNA-101 potently inhibits hepatocellular carcinoma in vivo by repressing multiple targets. PLoS Genet. 11, e1004873 (2015).
Zheng, F. et al. The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2. Gut 61, 278–289 (2012).
Acknowledgements
We thank V.N. Kim for pFlag-DROSHA, C. Basler for pFlag-DRBP76, Feng Zhang for pSpCas9(BB)-2A-GFP (PX458; Addgene plasmid # 48138), and members of the labs for helpful discussions. J.B. and G.S. were supported by ANRS and ERC, X.C. by CNRS, FRM FDT20111223640 and NSFC, M.H. and P.G. by ERC and L.B. by FRM. This work was supported by grants from the National Key R&D Program of China (no. 2017YFC1309001 and no. 2016YFC1302305) to D.X., ARC SFI20121205660, FRM DEQ20130326505 and ING20121226264, MSDAvenir and ERC CoG RNAmedTGS to R.K., Japan Society for the Promotion of Science Grant-in-aid for Young Scientists (B) 17K15601, Grant-in-aid for Scientific Research (C) 16K08590 to S.S. and NSFC 81602233 to X.C.
Author information
Authors and Affiliations
Contributions
J.B., X.C., G.S., M.C., X.Co., M.H., T.H., P.G., G.Y., Z.F., R.N.-S., W.R., S.R., O.D., L.B. and J.-G.J. conducted the experiments. J.B., G.S., X.C., S.S., D.X. and R.K. designed the experiments and D.X. and R.K. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Barbier, J., Chen, X., Sanchez, G. et al. An NF90/NF110-mediated feedback amplification loop regulates dicer expression and controls ovarian carcinoma progression. Cell Res 28, 556–571 (2018). https://doi.org/10.1038/s41422-018-0016-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41422-018-0016-8
This article is cited by
-
Chromatin-associated circRNA ciCRLF3(2) regulates cell differentiation blockage via activating non-homologous end joining–based DNA repair
Cell Death & Differentiation (2026)
-
Identification of antiviral RNAi regulators, ILF3/DHX9, recruit at ZIKV stem loop B to protect against ZIKV induced microcephaly
Nature Communications (2025)
-
pH- and Reduction-Responsive Supramolecular Hydrogels Based on Pluronic F127-Modified Hyaluronic Acid for the Targeted Delivery of Doxorubicin
Arabian Journal for Science and Engineering (2025)
-
MiR-383 sensitizes osteosarcoma cells to bortezomib treatment via down-regulating PSMB5
Molecular Biology Reports (2024)
-
LINC00893 inhibits the progression of prostate cancer through miR-3173-5p/SOCS3/JAK2/STAT3 pathway
Cancer Cell International (2022)