Abstract
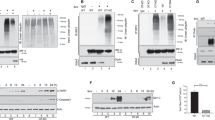
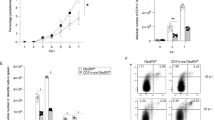
The activity and stability of the adapter protein MAVS (also known as VISA, Cardif and IPS-1), which critically mediates cellular antiviral responses, are extensively regulated by ubiquitination. However, the process whereby MAVS is deubiquitinated is unclear. Here, we report that the ovarian tumor family deubiquitinase 4 (OTUD4) targets MAVS for deubiquitination. Viral infection leads to the IRF3/7-dependent upregulation of OTUD4 which interacts with MAVS to remove K48-linked polyubiquitin chains, thereby maintaining MAVS stability and promoting innate antiviral signaling. Knockout or knockdown of OTUD4 impairs RNA virus-triggered activation of IRF3 and NF-κB, expression of their downstream target genes, and potentiates VSV replication in vitro and in vivo. Consistently, Cre-ER Otud4fl/fl or Lyz2-Cre Otud4fl/fl mice produce decreased levels of type I interferons and proinflammatory cytokines and exhibit increased sensitivity to VSV infection compared to their control littermates. In addition, reconstitution of MAVS into OTUD4-deficient cells restores virus-induced expression of downstream genes and cellular antiviral responses. Together, our findings uncover an essential role of OTUD4 in virus-triggered signaling and contribute to the understanding of deubiquitination-mediated regulation of innate antiviral responses.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Wu, J. & Chen, Z. J. Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol. 32, 461–488 (2014).
Pichlmair, A. et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5 ‘-phosphates. Science 314, 997–1001 (2006).
Hornung, V. et al. 5 ‘-triphosphate RNA is the ligand for RIG-I. Science 314, 994–997 (2006).
Myong, S. et al. Cytosolic viral sensor RIG-I is a 5 ‘-triphosphate-dependent translocase on double-stranded RNA. Science 323, 1070–1074 (2009).
Schlee, M. et al. Recognition of 5 ‘triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity 31, 25–34 (2009).
Kato, H. et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 205, 1601–1610 (2008).
Chiu, Y.-H., MacMillan, J. B. & Chen, Z. J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138, 576–591 (2009).
Ablasser, A. et al. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat. Immunol. 10, 1065–72 (2009).
Seth, R. B., Sun, L. J., Ea, C. K. & Chen, Z. J. J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappa B and IRF3. Cell 122, 669–682 (2005).
Hou, F. et al. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 146, 448–461 (2011).
Cai, X. et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell 156, 1207–1222 (2014).
Xu, L. G. et al. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol. Cell 19, 727–740 (2005).
Meylan, E. et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437, 1167–1172 (2005).
Kawai, T. et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6, 981–988 (2005).
Liu, S. et al. MAVS recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades. eLife 2, eLife.00785 (2013).
Fang, R. et al. MAVS activates TBK1 and IKK epsilon through TRAFs in NEMO dependent and independent manner. PLoS. Pathog. 13, e1006720 (2017).
Hemmi, H. et al. The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J. Exp. Med. 199, 1641–1650 (2004).
Perry, A. K., Chow, E. K., Goodnough, J. B., Yeh, W. C. & Cheng, G. Differential requirement for TANK-binding kinase-1 in type I interferon responses to toll-like receptor activation and viral infection. J. Exp. Med. 199, 1651–1658 (2004).
Kumar, H. et al. Essential role of IPS-1 in innate immune responses against RNA viruses. J. Exp. Med. 203, 1795–1803 (2006).
Sun, Q. M. et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity 24, 633–642 (2006).
Liu, S. et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 347, 1217–U1217 (2015).
Xiang, W. et al. PPM1A silences cytosolic RNA sensing and antiviral defense through direct dephosphorylation of MAVS and TBK1. Sci. Adv. 2, e1501889 (2016).
Liu, B. et al. The ubiquitin E3 ligase TRIM31 promotes aggregation and activation of the signaling adaptor MAVS through Lys63-linked polyubiquitination. Nat. Immunol. 18, 214–224 (2017).
Castanier, C. et al. MAVS ubiquitination by the E3 ligase TRIM25 and degradation by the proteasome is involved in type I interferon production after activation of the antiviral RIG-I-like receptors. BMC Biol. 10, 44 (2012).
Zhou, X., You, F., Chen, H. & Jiang, Z. Poly(C)-binding protein 1 (PCBP1) mediates housekeeping degradation of mitochondrial antiviral signaling (MAVS). Cell Res. 22, 717–727 (2012).
You, F. et al. PCBP2 mediates degradation of the adaptor MAVS via the HECT ubiquitin ligase AIP4. Nat. Immunol. 10, 1300–U1310 (2009).
Choi, Y. B., Shembade, N., Parvatiyar, K., Balachandran, S. & Harhaj, E. W. TAX1BP1 restrains virus-induced apoptosis by facilitating Itch-mediated degradation of the mitochondrial adaptor MAVS. Mol. Cell. Biol. 37, pii: e00422–16 (2017).
Zhong, B. et al. The E3 ubiquitin ligase RNF5 targets virus-induced signaling adaptor for ubiquitination and degradation. J. Immunol. 184, 6249–6255 (2010).
Jia, Y. et al. Negative regulation of MAVS-mediated innate immune response by PSMA7. J. Immunol. 183, 4241–4248 (2009).
Wang, Y., Tong, X. & Ye, X. Ndfip1 negatively regulates RIG-I-dependent immune signaling by enhancing E3 ligase Smurf1-mediated MAVS degradation. J. Immunol. 189, 5304–5313 (2012).
Pan, Y. et al. Smurf2 negatively modulates RIG-I-dependent antiviral response by targeting VISA/MAVS for ubiquitination and degradation. J. Immunol. 192, 4758–4764 (2014).
Yoo, Y.-S. et al. The mitochondrial ubiquitin ligase MARCH5 resolves MAVS aggregates during antiviral signalling. Nat. Communs 6, 8910 (2015).
Jin, S. et al. Tetherin suppresses type I interferon signaling by targeting MAVS for NDP52-mediated selective autophagic degradation in human cells. Mol. Cell 68, 308–322 (2017).
Zhang, L. et al. Induction of OTUD1 by RNA viruses potently inhibits innate immune responses by promoting degradation of the MAVS/TRAF3/TRAF6 signalosome. Plos Pathog. 14, e1007067 (2018).
Luo, W.-W. et al. iRhom2 is essential for innate immunity to RNA virus by antagonizing ER- and mitochondria-associated degradation of VISA. Plos Pathog. 13, e1006693 (2017).
Mevissen, T. E. T. & Komander, D. Mechanisms of deubiquitinase specificity and regulation. Annu. Rev. Biochem. 86, 159–192 (2017).
Tse, W. K. F. et al. Genome-wide loss-of-function analysis of deubiquitylating enzymes for zebrafish development. BMC Genom. 10, 637 (2009).
Tse, W. K. F., Jiang, Y.-J. & Wong, C. K. C. Zebrafish transforming growth factor-beta-stimulated clone 22 domain 3 (TSC22D3) plays critical roles in Bmp-dependent dorsoventral patterning via two deubiquitylating enzymes Usp15 and Otud4. Biochim Biophys. Acta1 830, 4584–4593 (2013).
Margolin, D. H. et al. Ataxia, dementia, and hypogonadotropism caused by disordered ubiquitination. N. Engl. J. Med. 368, 1992–2003 (2013).
Zhao, Y. et al. Noncanonical regulation of alkylation damage resistance by the OTUD4 deubiquitinase. EMBO J. 34, 1687–1703 (2015).
Mevissen, T. E. T. et al. OTU deubiquitinases reveal mechanisms of linkage specificity and enable ubiquitin chain restriction analysis. Cell 154, 169–184 (2013).
Zhao, Y. et al. OTUD4 is a phospho-activated K63 deubiquitinase that regulates MyD88-dependent signaling. Mol. Cell 69, 505–516 (2018).
Zhang, M. et al. USP18 recruits USP20 to promote innate antiviral response through deubiquitinating STING/MITA. Cell Res. 26, 1302–1319 (2016).
Sun, H. et al. USP13 negatively regulates antiviral responses by deubiquitinating STING. Nat. Communs 8, 15534 (2017).
Hameyer, D. et al. Toxicity of ligand-dependent Cre recombinases and generation of a conditional Cre deleter mouse allowing mosaic recombination in peripheral tissues. Physiol. Genom. 31, 32–41 (2007).
Aas, P. A. et al. Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature 421, 859–863 (2003).
Camini, F. C., da Silva Caetano, C. C., Almeida, L. T. & de Brito Magalhaes, C. L. Implications of oxidative stress on viral pathogenesis. Arch. Virol. 162, 907–917 (2017).
Allen, E. K. et al. SNP-mediated disruption of CTCF binding at the IFITM3 promoter is associated with risk of severe influenza in humans. Nat. Med. 23, 975–983 (2017).
Wang, C. et al. The E3 ubiquitin ligase Nrdp1 ‘preferentially’ promotes TLR-mediated production of type I interferon. Nat. Immunol. 10, 744–752 (2009).
Paz, S. et al. A functional C-terminal TRAF3-binding site in MAVS participates in positive and negative regulation of the IFN antiviral response. Cell Res. 21, 895–910 (2011).
Lei, C.-Q. et al. ECSIT bridges RIG-I-like receptors to VISA in Signaling events of innate antiviral responses. J. Innate Immun. 7, 153–164 (2015).
Zhang, X. et al. GP73 represses host innate immune response to promote virus replication by facilitating MAVS and TRAF6 degradation. PLoS. Pathog. 13, e1006321 (2017).
Wang, J. et al. Duck Tembusu virus nonstructural protein 1 antagonizes IFN-beta signaling pathways by targeting VISA. J. Immunol. 197, 4704–4713 (2016).
Zhong, B. et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29, 538–550 (2008).
Lu, B. et al. Induction of INKIT by viral infection negatively regulates antiviral responses through inhibiting phosphorylation of p65 and IRF3. Cell. Host. Microbe 22, 86–98 (2017).
Zhong, B. et al. Negative regulation of IL-17-mediated signaling and inflammation by the ubiquitin-specific protease USP25. Nat. Immunol. 13, 1110–1117 (2012).
Lin, D. et al. Induction of USP25 by viral infection promotes innate antiviral responses by mediating the stabilization of TRAF3 and TRAF6. Proc. Natl Acad. Sci. USA 112, 11324–113291 (2015).
Zhao, Y. et al. USP2a supports metastasis by tuning TGF-beta signaling. Cell Rep. 22, 2442–2454 (2018).
Ren, Y. et al. The type I interferon-IRF7 axis mediates transcriptional expression of Usp25 gene. J. Biol. Chem. 291, 13206–13215 (2016).
Acknowledgements
We would like to thank Drs. Hong-Bing Shu (Wuhan University) and Chen Dong (Tsinghua University) for reagents, members of Zhong lab and the core facilities of Medical Research Institute for technical help. This study was supported by grants from the Ministry of Science and Technology of China (2014CB542601), Natural Science Foundation of China (31521091, 31601131, 31671454 and 31622036), National Natural Science Foundation of Hubei Province (2018CFA016), Wuhan University (2042017kf0199 and 2042017kf0242) and State Key Laboratory of Veterinary Etiological Biology (SKLVEB2017KFKT004).
Author information
Authors and Affiliations
Contributions
B.Z. designed and supervised the study. T.L. designed and performed the major experiments; K.Y., L.Y., M.Z. and Z.C. helped with the animal studies; Z.Z. and Y.R. performed the ChIP analysis; Q.Z. provided reagents; B.Z., D.L. and T.L. wrote the paper. All authors analyzed data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Liuyu, T., Yu, K., Ye, L. et al. Induction of OTUD4 by viral infection promotes antiviral responses through deubiquitinating and stabilizing MAVS. Cell Res 29, 67–79 (2019). https://doi.org/10.1038/s41422-018-0107-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41422-018-0107-6
Keywords
This article is cited by
-
Old dogs-new tricks: multifaceted functions of MAVS beyond antivirus activity in human health and diseases
Cell & Bioscience (2025)
-
Deubiquitination enzyme USP35 negatively regulates MAVS signaling to inhibit anti-tumor immunity
Cell Death & Disease (2025)
-
CLK2 mediates IκBα-independent early termination of NF-κB activation by inducing cytoplasmic redistribution and degradation
Nature Communications (2024)
-
Deubiquitinating enzyme OTUD4 stabilizes RBM47 to induce ATF3 transcription: a novel mechanism underlying the restrained malignant properties of ccRCC cells
Apoptosis (2024)
-
Deubiquitylase YOD1 regulates CDK1 stability and drives triple-negative breast cancer tumorigenesis
Journal of Experimental & Clinical Cancer Research (2023)