Abstract
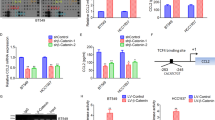
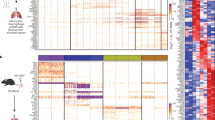
Macrophages have been suggested to contribute to constructing a cancer stem cell (CSC) niche. However, whether and how macrophages regulate the activity of CSCs through juxtacrine signaling are poorly understood. Here we report LSECtin, a transmembrane protein highly expressed on tumor-associated macrophages (TAMs), enhances stemness of breast cancer cells (BCCs). We identified BTN3A3, a B7 family member with previously unknown functions as the receptor for LSECtin on BCCs responsible for stemness-promoting effect of LSECtin. In mice bearing human tumor xenografts, either macrophage-specific ablation of LSECtin or silencing of BTN3A3 in BCCs decreased CSC frequency and tumor growth. Admixture of LSECtin-positive macrophages increased the tumorigenic activity of BCCs dependent on BTN3A3. Disruption of the LSECtin-BTN3A3 axis with BTN3A3-Fc or anti-BTN3A3 mAb has a therapeutic effect on breast cancer. These findings define a juxtacrine signaling mechanism by which TAMs promote cancer stemness. Targeting this axis in the CSC niche may provide potential therapies to breast cancer.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Plaks, V., Kong, N. & Werb, Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell. Stem. Cell. 16, 225–238 (2015).
Oskarsson, T., Batlle, E. & Massague, J. Metastatic stem cells: sources, niches, and vital pathways. Cell. Stem. Cell. 14, 306–321 (2014).
Qian, B. Z. & Pollard, J. W. Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39–51 (2010).
Mantovani, A., Marchesi, F., Malesci, A., Laghi, L. & Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14, 399–416 (2017).
Chen, J. et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 19, 541–555 (2011).
Liu, W. et al. Characterization of a novel C-type lectin-like gene, LSECtin: demonstration of carbohydrate binding and expression in sinusoidal endothelial cells of liver and lymph node. J. Biol. Chem. 279, 18748–18758 (2004).
Liu, B. et al. Liver sinusoidal endothelial cell lectin inhibits CTL-dependent virus clearance in mouse models of viral hepatitis. J. Immunol. 190, 4185–4195 (2013).
Tang, L. et al. Liver sinusoidal endothelial cell lectin, LSECtin, negatively regulates hepatic T-cell immune response. Gastroenterology 137, 1498–1508, e1491–1495 (2009).
Xu, F. et al. LSECtin expressed on melanoma cells promotes tumor progression by inhibiting antitumor T-cell responses. Cancer Res. 74, 3418–3428 (2014).
Dominguez-Soto, A. et al. The DC-SIGN-related lectin LSECtin mediates antigen capture and pathogen binding by human myeloid cells. Blood 109, 5337–5345 (2007).
Xue, J. et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–288 (2014).
Abeler-Dorner, L., Swamy, M., Williams, G., Hayday, A. C. & Bas, A. Butyrophilins: an emerging family of immune regulators. Trends Immunol. 33, 34–41 (2012).
Rhodes, D. A., Reith, W. & Trowsdale, J. Regulation of Immunity by Butyrophilins. Annu. Rev. Immunol. 34, 151–172 (2016).
Arnett, H. A. & Viney, J. L. Immune modulation by butyrophilins. Nat. Rev. Immunol. 14, 559–569 (2014).
Cubillos-Ruiz, J. R. et al. CD277 is a negative co-stimulatory molecule universally expressed by ovarian cancer microenvironmental cells. Oncotarget 1, 329–338 (2010).
Compte, E., Pontarotti, P., Collette, Y., Lopez, M. & Olive, D. Frontline: characterization of BT3 molecules belonging to the B7 family expressed on immune cells. Eur. J. Immunol. 34, 2089–2099 (2004).
Zhu, M. et al. Exome array analysis identifies variants in SPOCD1 and BTN3A2 that affect risk for gastric cancer. Gastroenterology 152, 2011–2021 (2017).
Peedicayil, A. et al. Risk of ovarian cancer and inherited variants in relapse-associated genes. PLoS One 5, e8884 (2010).
Ruffell, B. et al. Leukocyte composition of human breast cancer. Proc. Natl. Acad. Sci. USA 109, 2796–2801 (2012).
Franklin, R. A. et al. The cellular and molecular origin of tumor-associated macrophages. Science 344, 921–925 (2014).
Liu, D. et al. Comprehensive proteomics analysis reveals metabolic reprogramming of tumor-associated macrophages stimulated by the tumor microenvironment. J. Proteome Res. 16, 288–297 (2017).
Lu, H. et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat. Cell Biol. 16, 1105–1117 (2014).
Chen, Q., Zhang, X. H. & Massague, J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 20, 538–549 (2011).
Ben-Porath, I. et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 40, 499–507 (2008).
Uhlen, M. et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Marotta, L. L. et al. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(-) stem cell-like breast cancer cells in human tumors. J. Clin. Invest. 121, 2723–2735 (2011).
Zhou, B. et al. Erythropoietin promotes breast tumorigenesis through tumor-initiating cell self-renewal. J. Clin. Invest. 124, 553–563 (2014).
Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Vanharanta, S. & Massague, J. Origins of metastatic traits. Cancer Cell. 24, 410–421 (2013).
Liu, Y. R. et al. Comprehensive transcriptome profiling reveals multigene signatures in triple-negative breast cancer. Clin. Cancer Res. 22, 1653–1662 (2016).
Burstein, M. D. et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin. Cancer Res. 21, 1688–1698 (2015).
Peng, D. et al. Myeloid-derived suppressor cells endow stem-like qualities to breast cancer cells through IL6/STAT3 and NO/NOTCH cross-talk signaling. Cancer Res. 76, 3156–3165 (2016).
Cui, T. X. et al. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity 39, 611–621 (2013).
Li, W. et al. Aerobic glycolysis controls myeloid-derived suppressor cells and tumor immunity via a specific CEBPB isoform in triple-negative breast cancer. Cell. Metab. 28, 87–103 e106 (2018).
Pattabiraman, D. R. & Weinberg, R. A. Tackling the cancer stem cells - what challenges do they pose? Nat. Rev. Drug. Discov. 13, 497–512 (2014).
Dontu, G. et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 17, 1253–1270 (2003).
Acknowledgements
We thank Imaging Facility of National Center for Protein Sciences· Beijing (NCPSB) (Mrs. Ping Wu) for Microscopy Imaging, Animal Facility of NCPSB (Mr. Chen Qiu), Flow Cytometry Facility of NCPSB (Mr. Yunxiang Sun and Ms. Mingxin Zhao) for FACS. We thank Mr. Pumin Zhang and Mrs. Jin Peng for cell culturing, Mr. Lichun Tang for gene editing (National Center for Protein Sciences·Beijing), Mr Feng Xu for WB (National Center for Protein Sciences·Beijing). This work was supported by the National Natural Science Foundation (31570901), Beijing Science and Technology Program Foundation (Z141100000214015), National Key R&D Program of China (2018YFA0507500), State Key Laboratory of Proteomics Foundation (SKLP-K201504, SKLP-K201701) and National Basic Research Program of China (973 Program) (2014CBA02000). Project was also funded by China Postdoctoral Science Foundation (2018M633740).
Author contributions
LT and FH planned the project. DL, QL, XW, JW, NL, ZJ, XH, JL, JL and DZ carried out experimental work. PC, GP, and YT analyzed data. DL and LT wrote the paper. All authors discussed the results and commented on the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, D., Lu, Q., Wang, X. et al. LSECtin on tumor-associated macrophages enhances breast cancer stemness via interaction with its receptor BTN3A3. Cell Res 29, 365–378 (2019). https://doi.org/10.1038/s41422-019-0155-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41422-019-0155-6
This article is cited by
-
Tumor-associated macrophages remodel the suppressive tumor immune microenvironment and targeted therapy for immunotherapy
Journal of Experimental & Clinical Cancer Research (2025)
-
M2 macrophage-secreted KYNU promotes stemness remodeling and malignant behavior in endometrial cancer via the SOD2-mtROS-ERO1α-UPRER axis
Journal of Experimental & Clinical Cancer Research (2025)
-
Breast cancer: pathogenesis and treatments
Signal Transduction and Targeted Therapy (2025)
-
Tissue macrophages: origin, heterogenity, biological functions, diseases and therapeutic targets
Signal Transduction and Targeted Therapy (2025)
-
Tumour-associated macrophages serve as an acetate reservoir to drive hepatocellular carcinoma metastasis
Nature Metabolism (2025)