Abstract
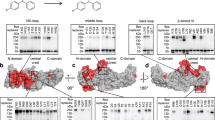
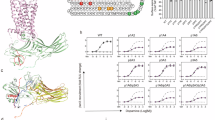
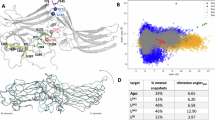
Arrestins comprise a family of signal regulators of G-protein-coupled receptors (GPCRs), which include arrestins 1 to 4. While arrestins 1 and 4 are visual arrestins dedicated to rhodopsin, arrestins 2 and 3 (Arr2 and Arr3) are β-arrestins known to regulate many nonvisual GPCRs. The dynamic and promiscuous coupling of Arr2 to nonvisual GPCRs has posed technical challenges to tackle the basis of arrestin binding to GPCRs. Here we report the structure of Arr2 in complex with neurotensin receptor 1 (NTSR1), which reveals an overall assembly that is strikingly different from the visual arrestin–rhodopsin complex by a 90° rotation of Arr2 relative to the receptor. In this new configuration, intracellular loop 3 (ICL3) and transmembrane helix 6 (TM6) of the receptor are oriented toward the N-terminal domain of the arrestin, making it possible for GPCRs that lack the C-terminal tail to couple Arr2 through their ICL3. Molecular dynamics simulation and crosslinking data further support the assembly of the Arr2‒NTSR1 complex. Sequence analysis and homology modeling suggest that the Arr2‒NTSR1 complex structure may provide an alternative template for modeling arrestin–GPCR interactions.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Shukla, A. K., Xiao, K. & Lefkowitz, R. J. Emerging paradigms of beta-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem. Sci. 36, 457–469 (2011).
Ritter, S. L. & Hall, R. A. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat. Rev. Mol. Cell Biol. 10, 819–830 (2009).
Gurevich, V. V. & Gurevich, E. V. Molecular mechanisms of GPCR signaling: a structural perspective. Int. J. Mol. Sci. 18, https://doi.org/10.3390/ijms18122519 (2017).
Gurevich, V. V. & Gurevich, E. V. Structural determinants of arrestin functions. Prog. Mol. Biol. Transl. Sci. 118, 57–92 (2013).
Kang, D. S., Tian, X. & Benovic, J. L. Role of beta-arrestins and arrestin domain-containing proteins in G protein-coupled receptor trafficking. Curr. Opin. Cell Biol. 27, 63–71 (2014).
Zhou, X. E., Melcher, K. & Xu, H. E. Understanding the GPCR biased signaling through G protein and arrestin complex structures. Curr. Opin. Struct. Biol. 45, 150–159 (2017).
Kang, Y. et al. Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature 523, 561–567 (2015).
Zhou, X. E. et al. X-ray laser diffraction for structure determination of the rhodopsin–arrestin complex. Sci. Data 3, 160021 (2016).
Zhou, X. E. et al. Identification of phosphorylation codes for arrestin recruitment by G protein-coupled receptors. Cell 170, 457–469 e413 (2017).
Zhou, X. E., Melcher, K. & Xu, H. E. Structural biology of G protein-coupled receptor signaling complexes. Protein Sci. 28, 487–501 (2019).
Shukla, A. K. et al. Visualization of arrestin recruitment by a G-protein-coupled receptor. Nature 512, 218–222 (2014).
Tanaka, K., Masu, M. & Nakanishi, S. Structure and functional expression of the cloned rat neurotensin receptor. Neuron 4, 847–854 (1990).
Kitabgi, P. Targeting neurotensin receptors with agonists and antagonists for therapeutic purposes. Curr. Opin. Drug Discov. Dev. 5, 764–776 (2002).
White, J. F. et al. Structure of the agonist-bound neurotensin receptor. Nature 490, 508–513 (2012).
Krumm, B. E., White, J. F., Shah, P. & Grisshammer, R. Structural prerequisites for G-protein activation by the neurotensin receptor. Nat. Commun. 6, 7895 (2015).
Krumm, B. E. et al. Structure and dynamics of a constitutively active neurotensin receptor. Sci. Rep. 6, 38564 (2016).
Dixon, A. S. et al. NanoLuc complementation reporter optimized for accurate measurement of protein interactions in cells. ACS Chem. Biol. 11, 400–408 (2016).
Gurevich, V. V. The selectivity of visual arrestin for light-activated phosphorhodopsin is controlled by multiple nonredundant mechanisms. J. Biol. Chem. 273, 15501–15506 (1998).
Celver, J., Vishnivetskiy, S. A., Chavkin, C. & Gurevich, V. V. Conservation of the phosphate-sensitive elements in the arrestin family of proteins. J. Biol. Chem. 277, 9043–9048 (2002).
Barnea, G. et al. The genetic design of signaling cascades to record receptor activation. Proc. Natl Acad. Sci. USA 105, 64–69 (2008).
Peddibhotla, S. et al. Discovery of ML314, a brain penetrant non-peptidic beta-arrestin biased agonist of the neurotensin NTR1 receptor. ACS Med. Chem. Lett. 4, 846–851 (2013).
Barak, L. S. et al. ML314: a biased neurotensin receptor ligand for methamphetamine abuse. ACS Chem. Biol. 11, 1880–1890 (2016).
Shukla, A. K. et al. Structure of active beta-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature 497, 137–141 (2013).
Alexandrov, A. I., Mileni, M., Chien, E. Y. T., Hanson, M. A. & Stevens, R. C. Microscale fluorescent thermal stability assay for membrane proteins. Structure 16, 351–359 (2008).
Wang, R. Y. et al. Automated structure refinement of macromolecular assemblies from cryo-EM maps using Rosetta. Elife 5, https://doi.org/10.7554/eLife.17219 (2016).
Lally, C. C., Bauer, B., Selent, J. & Sommer, M. E. C-edge loops of arrestin function as a membrane anchor. Nat. Commun. 8, 14258 (2017).
Sommer, M. E., Hofmann, K. P. & Heck, M. Distinct loops in arrestin differentially regulate ligand binding within the GPCR opsin. Nat. Commun. 3, 995 (2012).
Janoshazi, A. et al. Modified receptor internalization upon coexpression of 5-HT1B receptor and 5-HT2B receptors. Mol. Pharmacol. 71, 1463–1474 (2007).
Wang, C. et al. Structural basis for molecular recognition at serotonin receptors. Science 340, 610–614 (2013).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7, https://doi.org/10.7554/eLife.42166 (2018).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Pettersen, E. F. et al. UCSF chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Sect. D 66, 213–221 (2010).
DiMaio, F. et al. Improved low-resolution crystallographic refinement with Phenix and Rosetta. Nat. Methods 10, 1102–1104 (2013).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. Sect. D 66, 12–21 (2010).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017).
Case, D. A. et al. AMBER 2018 (Unviersity of California, San Francisco, 2018).
Lomize, M. A., Lomize, A. L., Pogozheva, I. D. & Mosberg, H. I. OPM: orientations of proteins in membranes database. Bioinformatics 22, 623–625 (2006).
Eswar, N. et al. Comparative protein structure modeling using Modeller. Curr. Protoc. Bioinformatics Chapter 5, Unit-5–6, 10.1002/0471250953.bi0506s15 (2006).
McGibbon, R. T. et al. MDTraj: a modern open library for the analysis of molecular dynamics trajectories. Biophys. J. 109, 1528–1532 (2015).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
RoeD. R. & Cheatham, T. EIII. PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 9, 3084–3095 (2013).
Acknowledgements
The cryo-EM data were collected at Cryo-Electron Microscopy Research Center, Shanghai Institute of Material Medica. We are also grateful to the staff of the National Center for Protein Science (Shanghai) Electron Microscopy facility for instrument support. We thank the Institutional Technology Service Center of Shanghai Institute of Materia Medica, Chinese Academy of Sciences for technical assistance in mass spectrometry experiments and analysis. This work was partially supported by Ministry of Science and Technology (China) grants 2012ZX09301001, 2012CB910403, 2013CB910600, XDB08020303, and 2013ZX09507001 (to H.E.X.); Van Andel Research Institute (K.M. and H.E.X.); National Institutes of Health grants (GM127710 to H.E.X.), the 100 Talents Program of the Chinese Academy of Sciences (to X.Y.); Chinese Academy of Sciences grant (XDA12010317 to X.Y.); Natural Science Foundation of Shanghai (18ZR1447700 to X.Y.); Shanghai Sailing Program (19YF1456800 to Z.L.); China Postdoctoral Science Foundation (2019M651622 to Z.L.), National Basic Research Program of China (2017YFA0503503), NSFC (31670754), CAS (DSS-WXJZ-2018-0002, CAS-SSRC-YH-2015-01), and the CAS Major Science and Technology Infrastructure Open Research Projects to Y.C.
Author information
Authors and Affiliations
Contributions
W.Y. designed the expression constructs, purified the Arr2‒NTSR1 complex, prepared the final samples for negative stain and data collection toward the structures, design functional assays, performed disulfide crosslinking, and participated in figure and manuscript preparation. Z.L. performed cryo-EM data collection, processing, map refinement and figure preparation; M.J. and Y.C. performed cryo-EM sample screening, initial reconstruction and figure preparation; Y.-L.Y. and Y.Y. performed cell-based functional experiments; P.W.d.W. performed MD simulations and figure preparation; K.P., X.G., Y.H., and X.W. performed construct design and disulfide crosslinking; Y.Z. participated in EM map interpretation and figure preparation; J.G. and H.Z. performed mass spectrometry phosphorylation identification; K.M. and Y.J. participated in experimental design and manuscript editing; X.E.Z. built and refined the structure models, prepared figures and wrote the manuscript; X.Y. designed cryo-EM data collection and processing strategy, performed data processing and map refinement, and participated in manuscript editing; H.E.X. conceived and supervised the project, analyzed the structures, and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Yin, W., Li, Z., Jin, M. et al. A complex structure of arrestin-2 bound to a G protein-coupled receptor. Cell Res 29, 971–983 (2019). https://doi.org/10.1038/s41422-019-0256-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41422-019-0256-2
This article is cited by
-
Distinct membrane binding properties of the two non-visual arrestins
Communications Biology (2026)
-
Strategic advances for cryo-EM structural studies of small (<100 kDa) GPCRs
Communications Biology (2026)
-
A structural overview of G-protein-coupled receptors in neurological disorders
Acta Pharmacologica Sinica (2026)
-
Identification of a novel nonsense variant in ARR3 in a family with early-onset high myopia
Genes & Genomics (2026)
-
Orchestrating NTSR1 signaling from the interface
Cell Research (2025)