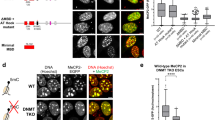
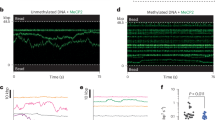
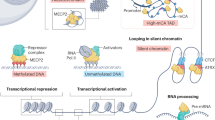
Abstract
Rett syndrome (RTT), a severe postnatal neurodevelopmental disorder, is caused by mutations in the X-linked gene encoding methyl-CpG-binding protein 2 (MeCP2). MeCP2 is a chromatin organizer regulating gene expression. RTT-causing mutations have been shown to affect this function. However, the mechanism by which MeCP2 organizes chromatin is unclear. In this study, we found that MeCP2 can induce compaction and liquid–liquid phase separation of nucleosomal arrays in vitro, and DNA methylation further enhances formation of chromatin condensates by MeCP2. Interestingly, RTT-causing mutations compromise MeCP2-mediated chromatin phase separation, while benign variants have little effect on this process. Moreover, MeCP2 competes with linker histone H1 to form mutually exclusive chromatin condensates in vitro and distinct heterochromatin foci in vivo. RTT-causing mutations reduce or even abolish the ability of MeCP2 to compete with histone H1 and to form chromatin condensates. Together, our results identify a novel mechanism by which phase separation underlies MeCP2-mediated heterochromatin formation and reveal the potential link between this process and the pathology of RTT.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lyst, M. J. & Bird, A. Rett syndrome: a complex disorder with simple roots. Nat. Rev. Genet. 16, 261–275 (2015).
Hagberg, B., Aicardi, J., Dias, K. & Ramos, O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: report of 35 cases. Ann. Neurol. 14, 471–479 (1983).
Armstrong, D. D. Neuropathology of Rett syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 8, 72–76 (2002).
Amir, R. E. et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 23, 185–188 (1999).
Lewis, J. D. et al. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell 69, 905–914 (1992).
Meehan, R. R., Lewis, J. D. & Bird, A. P. Characterization of MeCP2, a vertebrate DNA binding protein with affinity for methylated DNA. Nucleic Acids Res. 20, 5085–5092 (1992).
Klose, R. J. et al. DNA binding selectivity of MeCP2 due to a requirement for A/T sequences adjacent to methyl-CpG. Mol. Cell 19, 667–678 (2005).
Nan, X., Campoy, F. J. & Bird, A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell 88, 471–481 (1997).
Thambirajah, A. A. et al. MeCP2 binds to nucleosome free (linker DNA) regions and to H3K9/H3K27 methylated nucleosomes in the brain. Nucleic Acids Res. 40, 2884–2897 (2012).
Skene, P. J. et al. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol. Cell 37, 457–468 (2010).
Jones, P. L. et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 19, 187 (1998).
Nan, X. et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393, 386–389 (1998).
Wade, P. A. Methyl CpG-binding proteins and transcriptional repression. BioEssays 23, 1131–1137 (2001).
Koerner, M. V. et al. Toxicity of overexpressed MeCP2 is independent of HDAC3 activity. Genes Dev. 32, 1514–1524 (2018).
Nikitina, T. et al. Multiple modes of interaction between the methylated DNA binding protein MeCP2 and chromatin. Mol. Cell. Biol. 27, 864–877 (2007).
Georgel, P. T. et al. Chromatin compaction by human MeCP2. J. Biol. Chem. 278, 32181–32188 (2003).
Ghosh, R. P. et al. Unique physical properties and interactions of the domains of methylated DNA binding protein 2. Biochemistry 49, 4395–4410 (2010).
Wang, L. et al. Histone modifications regulate chromatin compartmentalization by contributing to a phase separation mechanism. Mol. Cell 76, 646–659 (2019).
Gibson, B. A. et al. Organization of chromatin by intrinsic and regulated phase separation. Cell 179, 470–484.e421 (2019).
Larson, A. G. et al. Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature 547, 236–240 (2017).
Larson, A. G. & Narlikar, G. J. The role of phase separation in heterochromatin formation, function, and regulation. Biochemistry 57, 2540–2548 (2018).
Strom, A. R. et al. Phase separation drives heterochromatin domain formation. Nature 547, 241–245 (2017).
Brero, A. et al. Methyl CpG-binding proteins induce large-scale chromatin reorganization during terminal differentiation. J. Cell Biol. 169, 733–743 (2005).
Agarwal, N. et al. MeCP2 Rett mutations affect large scale chromatin organization. Hum. Mol. Genet. 20, 4187–4195 (2011).
Agarwal, N. et al. MeCP2 interacts with HP1 and modulates its heterochromatin association during myogenic differentiation. Nucleic Acids Res. 35, 5402–5408 (2007).
Guy, J., Cheval, H., Selfridge, J. & Bird, A. The role of MeCP2 in the brain. Annu. Rev. Cell Dev. Biol. 27, 631–652 (2011).
Baker, S. A. et al. An AT-hook domain in MeCP2 determines the clinical course of Rett syndrome and related disorders. Cell 152, 984–996 (2013).
Sperlazza, M. J., Bilinovich, S. M., Sinanan, L. M., Javier, F. R. & Williams, D. C. Jr. Structural basis of MeCP2 distribution on Non-CpG methylated and hydroxymethylated DNA. J. Mol. Biol. 429, 1581–1594 (2017).
Damen, D. & Heumann, R. MeCP2 phosphorylation in the brain: from transcription to behavior. Biol. Chem. 394, 1595–1605 (2013).
Mellen, M., Ayata, P., Dewell, S., Kriaucionis, S. & Heintz, N. MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system. Cell 151, 1417–1430 (2012).
Ghosh, R. P., Horowitz-Scherer, R. A., Nikitina, T., Gierasch, L. M. & Woodcock, C. L. Rett syndrome-causing mutations in human MeCP2 result in diverse structural changes that impact folding and DNA interactions. J. Biol. Chem. 283, 20523–20534 (2008).
Casas-Delucchi, C. S., Becker, A., Bolius, J. J. & Cardoso, M. C. Targeted manipulation of heterochromatin rescues MeCP2 Rett mutants and re-establishes higher order chromatin organization. Nucleic Acids Res. 40, e176 (2012).
Krishnaraj, R., Ho, G. & Christodoulou, J. RettBASE: Rett syndrome database update. Hum. Mutat. 38, 922–931 (2017).
Lombardi, L. M., Baker, S. A. & Zoghbi, H. Y. MECP2 disorders: from the clinic to mice and back. J. Clin. Investig. 125, 2914–2923 (2015).
Shah, R. R. & Bird, A. P. MeCP2 mutations: progress towards understanding and treating Rett syndrome. Genome Med. 9, 17 (2017).
Yu, D., Sakurai, F. & Corey, D. R. Clonal Rett syndrome cell lines to test compounds for activation of wild-type MeCP2 expression. Bioorg. Med. Chem. Lett. 21, 5202–5205 (2011).
Ghosh, R. P., Horowitz-Scherer, R. A., Nikitina, T., Shlyakhtenko, L. S. & Woodcock, C. L. MeCP2 binds cooperatively to its substrate and competes with histone H1 for chromatin binding sites. Mol. Cell. Biol. 30, 4656–4670 (2010).
Tillotson, R. & Bird, A. The molecular basis of MeCP2 function in the brain. J. Mol. Biol. https://doi.org/10.1016/j.jmb.2019.10.004 (2019).
Lyst, M. J. et al. Rett syndrome mutations abolish the interaction of MeCP2 with the NCoR/SMRT co-repressor. Nat. Neurosci. 16, 898–902 (2013).
Heckman, L. D., Chahrour, M. H. & Zoghbi, H. Y. Rett-causing mutations reveal two domains critical for MeCP2 function and for toxicity in MECP2 duplication syndrome mice. Elife 3, https://doi.org/10.7554/eLife.02676 (2014).
Nikitina, T. et al. MeCP2-chromatin interactions include the formation of chromatosome-like structures and are altered in mutations causing Rett syndrome. J. Biol. Chem. 282, 28237–28245 (2007).
Turner, A. L. et al. Highly disordered histone H1-DNA model complexes and their condensates. Proc. Natl. Acad. Sci. USA 115, 11964–11969 (2018).
Feric, M. et al. Coexisting liquid phases underlie nucleolar subcompartments. Cell 165, 1686–1697 (2016).
Putnam, A., Cassani, M., Smith, J. & Seydoux, G. A gel phase promotes condensation of liquid P granules in Caenorhabditis elegans embryos. Nat. Struct. Mol. Biol. 26, 220–226 (2019).
Gibbs, E. B. & Kriwacki, R. W. Linker histones as liquid-like glue for chromatin. Proc. Natl. Acad. Sci. USA 115, 11868–11870 (2018).
Kumar, A. et al. Analysis of protein domains and Rett syndrome mutations indicate that multiple regions influence chromatin-binding dynamics of the chromatin-associated protein MECP2 in vivo. J. Cell Sci. 121, 1128–1137 (2008).
Horike, S. [How the methyl-CpG binding protein-related epigenetic disease turns on the genes that produce its symptoms]. Tanpakushitsu Kakusan Koso 50, 978–984 (2005).
Mohan, A. et al. Analysis of molecular recognition features (MoRFs). J. Mol. Biol. 362, 1043–1059 (2006).
Lever, M. A., Th’ng, J. P., Sun, X. & Hendzel, M. J. Rapid exchange of histone H1.1 on chromatin in living human cells. Nature 408, 873–876 (2000).
Misteli, T., Gunjan, A., Hock, R., Bustin, M. & Brown, D. T. Dynamic binding of histone H1 to chromatin in living cells. Nature 408, 877–881 (2000).
Akhmanova, A., Verkerk, T., Langeveld, A., Grosveld, F. & Galjart, N. Characterisation of transcriptionally active and inactive chromatin domains in neurons. J. Cell Sci. 113, 4463–4474 (2000).
Della Ragione, F., Vacca, M., Fioriniello, S., Pepe, G. & D’Esposito, M. MECP2, a multi-talented modulator of chromatin architecture. Brief. Funct. Genom. 15, 420–431 (2016).
Pande, A. et al. Decrease in protein solubility and cataract formation caused by the Pro23 to Thr mutation in human gamma D-crystallin. Biochemistry 44, 2491–2500 (2005).
Dorsaz, N., Thurston, G. M., Stradner, A., Schurtenberger, P. & Foffi, G. Phase separation in binary eye lens protein mixtures. Soft Matter 7, 1763–1776 (2011).
Li, G. et al. Highly compacted chromatin formed in vitro reflects the dynamics of transcription activation in vivo. Mol. Cell 38, 41–53 (2010).
Dyer, P. N. et al. Reconstitution of nucleosome core particles from recombinant histones and DNA. Methods Enzymol. 375, 23–44 (2004).
Song, F. et al. Cryo-EM study of the chromatin fiber reveals a double helix twisted by tetranucleosomal units. Science 344, 376–380 (2014).
Chen, P. et al. H3.3 actively marks enhancers and primes gene transcription via opening higher-ordered chromatin. Genes Dev. 27, 2109–2124 (2013).
Li, W. et al. FACT remodels the tetranucleosomal unit of chromatin fibers for gene transcription. Mol. Cell 64, 120–133 (2016).
Modzelewski, A. J. et al. Efficient mouse genome engineering by CRISPR-EZ technology. Nat. Protoc. 13, 1253–1274 (2018).
Chen, Z. L. et al. A high-speed search engine pLink 2 with systematic evaluation for proteome-scale identification of cross-linked peptides. Nat. Commun. 10, 3404 (2019).
Acknowledgements
We thank Dr Xiaoqun Wang for helping us to get mouse brain slices, Dr Keping Hu for providing the MeCP2 plasmid as a gift, Master Ting Yao for her help in analytical ultracentrifugation experiments, and Dr Zhaolan Zhou for critical reading and discussion with our manuscript. This work was supported by grants from the Ministry of Science and Technology of China (2017YFA0504202 to G.L.; 2019YFA0508403 to P.L.; 2018YFE0203302 to P.C.), the National Natural Science Foundation of China (31525013, 31630041 and 31521002 to G.L.; 31871443 to P.L.; 31871290 to P.C.). The work was also supported by the Chinese Academy of Sciences (CAS) Strategic Priority Research Program (XDB19040202), the CAS Key Research Program on Frontier Science (QYZDY-SSW-SMC020) and an HHMI International Research Scholar grant (55008737) to G.L. We are particularly grateful to the SLSTU-Nikon Biological Imaging Center (Tsinghua university) for assistance with NIS-Elements AR Analysis, and Center for Bio-imaging & Core Facility for Protein Sciences (Institute of Biophysics, Chinese Academy of Sciences) for fluorescence and electron microscopy imaging data collection.
Author information
Authors and Affiliations
Contributions
L.W. carried out phase separation experiments and composed the figures. M.H. was responsible for AUC, EM images and cell fluorescence images. M.-Q.Z. performed CXMS experiments. J.Z. and L.H. generated the mice with the MeCP2 R106W mutation. D.W. helped with protein purification. Y.W. performed the statistical analysis of clinical data. Y.L. took part in the plasmid construction. P.C. helped with CXMS experiments and guided nucleosome assembly experiments. X.B. helped to discuss the project. M.-Q.D. guided CXMS experiments and data interpretation. G.L. and P.L. conceived and supervised the project, analyzed the data and wrote the manuscript with L.W. and M.H.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, L., Hu, M., Zuo, MQ. et al. Rett syndrome-causing mutations compromise MeCP2-mediated liquid–liquid phase separation of chromatin. Cell Res 30, 393–407 (2020). https://doi.org/10.1038/s41422-020-0288-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41422-020-0288-7
This article is cited by
-
Targeting redox-sensitive MBD2–NuRD condensate in cancer cells
Nature Cell Biology (2025)
-
Exploring the complexity of MECP2 function in Rett syndrome
Nature Reviews Neuroscience (2025)
-
Rett syndrome: advances in Understanding MeCP2 function, potential gene therapies, and public health implications
Molecular Biology Reports (2025)
-
Emerging roles of liquid-liquid phase separation in liver innate immunity
Cell Communication and Signaling (2024)
-
Liquid–liquid phase separation of H3K27me3 reader BP1 regulates transcriptional repression
Genome Biology (2024)