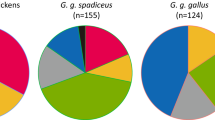Abstract
Despite the substantial role that chickens have played in human societies across the world, both the geographic and temporal origins of their domestication remain controversial. To address this issue, we analyzed 863 genomes from a worldwide sampling of chickens and representatives of all four species of wild jungle fowl and each of the five subspecies of red jungle fowl (RJF). Our study suggests that domestic chickens were initially derived from the RJF subspecies Gallus gallus spadiceus whose present-day distribution is predominantly in southwestern China, northern Thailand and Myanmar. Following their domestication, chickens were translocated across Southeast and South Asia where they interbred locally with both RJF subspecies and other jungle fowl species. In addition, our results show that the White Leghorn chicken breed possesses a mosaic of divergent ancestries inherited from other subspecies of RJF. Despite the strong episodic gene flow from geographically divergent lineages of jungle fowls, our analyses show that domestic chickens undergo genetic adaptations that underlie their unique behavioral, morphological and reproductive traits. Our study provides novel insights into the evolutionary history of domestic chickens and a valuable resource to facilitate ongoing genetic and functional investigations of the world’s most numerous domestic animal.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The raw sequencing data, alignment BAM files, and genotypes (VCF) have been deposited in the ChickenSD database (http://bigd.big.ac.cn/chickensd/).
Change history
20 July 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Lawler, A. Why did the chicken cross the world? Atria Books, New York (2014).
Larson, G. & Fuller, D. Q. The evolution of animal domestication. Annu. Rev. Ecol. Evol. Syst. 45, 115–136 (2014).
Peters, J. et al. Questioning new answers regarding Holocene chicken domestication in China. Proc. Natl Acad. Sci. USA 112, E2415 (2015).
Tixier-Boichard, M., Bed’hom, B. & Rognon, X. Chicken domestication: from archeology to genomics. C. R. Biol. 334, 197–204 (2011).
Miao, Y. W. et al. Chicken domestication: an updated perspective based on mitochondrial genomes. Heredity 110, 277–282 (2013).
Liu, Y. P. et al. Multiple maternal origins of chickens: out of the Asian jungles. Mol. Phylogenet. Evol. 38, 12–19 (2006).
Fumihito, A. et al. Monophyletic origin and unique dispersal patterns of domestic fowls. Proc. Natl Acad. Sci. USA 93, 6792–6795 (1996).
Lawler, A. Animal domestication. In search of the wild chicken. Science 338, 1020–1024 (2012).
Peters, J., Lebrasseur, O., Deng, H. & Larson, G. Holocene cultural history of red jungle fowl (Gallus gallus) and its domestic descendant in East Asia. Quat. Sci. Rev. 142, 102–119 (2016).
Eda, M. et al. Reevaluation of early Holocene chicken domestication in northern China. J. Archaeol. Sci. 67, 25–31 (2016).
Girdland Flink, L. et al. Establishing the validity of domestication genes using DNA from ancient chickens. Proc. Natl Acad. Sci. USA 111, 6184–6189 (2014).
Wang, G. D., Xie, H. B., Peng, M. S., Irwin, D. & Zhang, Y. P. Domestication genomics: evidence from animals. Annu. Rev. Anim. Biosci. 2, 65–84 (2014).
Larson, G. & Burger, J. A population genetics view of animal domestication. Trends Genet. 29, 197–205 (2013).
Wang, G. D. et al. Out of southern East Asia: the natural history of domestic dogs across the world. Cell Res. 26, 21–33 (2016).
Ní Leathlobhair, M. et al. The evolutionary history of dogs in the Americas. Science 361, 81–85 (2018).
Frantz, L. A. et al. Genomic and archaeological evidence suggest a dual origin of domestic dogs. Science 352, 1228–1231 (2016).
Chen, N. et al. Whole-genome resequencing reveals world-wide ancestry and adaptive introgression events of domesticated cattle in East Asia. Nat. Commun. 9, 2337 (2018).
Fages, A. et al. Tracking five millennia of horse management with extensive ancient genome time series. Cell 177, 1419–1435 (2019).
Flowers, J. M. et al. Cross-species hybridization and the origin of North African date palms. Proc. Natl Acad. Sci. USA 116, 1651–1658 (2019).
Li, D. et al. Population genomics identifies patterns of genetic diversity and selection in chicken. BMC Genom. 20, 263 (2019).
Rubin, C. J. et al. Whole-genome resequencing reveals loci under selection during chicken domestication. Nature 464, 587–591 (2010).
Wang, M. S. et al. An evolutionary genomic perspective on the breeding of dwarf chickens. Mol. Biol. Evol. 34, 3081–3088 (2017).
Guo, Y. et al. A complex structural variation on chromosome 27 leads to the ectopic expression of HOXB8 and the Muffs and beard phenotype in chickens. PLoS Genet. 12, e1006071 (2016).
Wang, M. S. et al. Positive selection rather than relaxation of functional constraint drives the evolution of vision during chicken domestication. Cell Res. 26, 556–573 (2016).
Guo, X. et al. Whole-genome resequencing of Xishuangbanna fighting chicken to identify signatures of selection. Genet. Sel. Evol. 48, 62 (2016).
Wang, M. S. et al. Genomic analyses reveal potential independent adaptation to high altitude in Tibetan chickens. Mol. Biol. Evol. 32, 1880–1889 (2015).
Wang, M. S. et al. Comparative population genomics reveals genetic basis underlying body size of domestic chickens. J. Mol. Cell. Biol. 8, 542–552 (2016).
Yi, G. et al. Genome-wide patterns of copy number variation in the diversified chicken genomes using next-generation sequencing. BMC Genom. 15, 962 (2014).
Fan, W. L. et al. Genome-wide patterns of genetic variation in two domestic chickens. Genome Biol. Evol. 5, 1376–1392 (2013).
Huang, X. H. et al. Was chicken domesticated in northern China? New evidence from mitochondrial genomes. Sci. Bull. 63, 743–746 (2018).
Alexander, D. H., Novembre, J. & Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19, 1655–1664 (2009).
Pickrell, J. K. & Pritchard, J. K. Inference of population splits and mixtures from genome-wide allele frequency data. PLoS Genet. 8, e1002967 (2012).
Patterson, N. et al. Ancient admixture in human history. Genetics 192, 1065–1093 (2012).
Schiffels, S. & Durbin, R. Inferring human population size and separation history from multiple genome sequences. Nat. Genet. 46, 919–925 (2014).
Nam, K. et al. Molecular evolution of genes in avian genomes. Genome Biol. 11, R68 (2010).
Collias, N. E. & Saichuae, P. Ecology of the red jungle fowl in Thailand and Malaya with reference to the origin of domestication. Nat. Hist. Bull. Siam. Soc. 22, 189–209 (1967).
Xiang, H. et al. Early Holocene chicken domestication in northern China. Proc. Natl Acad. Sci. USA 111, 17564–17569 (2014).
West, B. & Zhou, B. X. Did chickens go North? New evidence for domestication. J. Archaeol. Sci. 15, 515–533 (1988).
Brisbin, A. et al. PCAdmix: principal components-based assignment of ancestry along each chromosome in individuals with admixed ancestry from two or more populations. Hum. Biol. 84, 343–364 (2012).
Excoffier, L., Dupanloup, I., Huerta-Sanchez, E., Sousa, V. C. & Foll, M. Robust demographic inference from genomic and SNP data. PLoS Genet. 9, e1003905 (2013).
Eriksson, J. et al. Identification of the yellow skin gene reveals a hybrid origin of the domestic chicken. PLoS Genet. 4, e1000010 (2008).
Lawal, R. A. et al. The wild species genome ancestry of domestic chickens. BMC Biol. 18, 13 (2020).
Browning, B. L. & Browning, S. R. Genotype imputation with millions of reference samples. Am. J. Hum. Genet. 98, 116–126 (2016).
Bosse, M. et al. Genomic analysis reveals selection for Asian genes in European pigs following human-mediated introgression. Nat. Commun. 5, 4392 (2014).
Shriver, M. D. et al. The genomic distribution of population substructure in four populations using 8,525 autosomal SNPs. Hum. Genom. 1, 274–286 (2004).
Nei, M. & Li, W. H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl Acad. Sci. USA 76, 5269–5273 (1979).
Librado, P. et al. Ancient genomic changes associated with domestication of the horse. Science 356, 442–445 (2017).
Rohner, N. et al. Duplication of fgfr1 permits Fgf signaling to serve as a target for selection during domestication. Curr. Biol. 19, 1642–1647 (2009).
Atikuzzaman, M. et al. Selection for higher fertility reflects in the seminal fluid proteome of modern domestic chicken. Comp. Biochem. Physiol. Part D Genom. Proteom. 21, 27–40 (2017).
Schutz, K. E. et al. Major growth QTLs in fowl are related to fearful behavior: possible genetic links between fear responses and production traits in a red junglefowl x White Leghorn intercross. Behav. Genet. 34, 121–130 (2004).
Schutz, K. et al. QTL analysis of a red junglefowl x White Leghorn intercross reveals trade-off in resource allocation between behavior and production traits. Behav. Genet. 32, 423–433 (2002).
Bédécarrats, Y. G., Shimizu, M. & Guémené, D. Gonadotropin releasing hormones and their receptors in avian species. J. Poult. Sci. 43, 199–214 (2006).
Sharp, P. J. et al. Physiological roles of chicken LHRH-I and -II in the control of gonadotrophin release in the domestic chicken. J. Endocrinol. 124, 291–299 (1990).
Marques, P. et al. Physiology of GNRH and gonadotropin secretion. MDText.com, Inc. South Dartmouth, Massachusetts (2000).
Gandhi, R. et al. The Drosophila kinesin-like protein KLP67A is essential for mitotic and male meiotic spindle assembly. Mol. Biol. Cell 15, 121–131 (2004).
Liu, X. S. et al. Germinal cell aplasia in Kif18a mutant male mice due to impaired chromosome congression and dysregulated BubR1 and CENP-E. Genes Cancer 1, 26–39 (2010).
Loog, L. et al. Inferring allele frequency trajectories from ancient DNA indicates that selection on a chicken gene coincided with changes in medieval husbandry practices. Mol. Biol. Evol. 34, 1981–1990 (2017).
Qanbari, S. et al. Genetics of adaptation in modern chicken. PLoS Genet. 15, e1007989 (2019).
Ye, W. et al. Early middle Holocene climate oscillations recorded in the Beihuqiao Core, Yuhang, Zhejiang Province, China. J. Paleolimnol. 59, 263–278 (2017).
Wu, M. S. et al. Holocene hydrological and sea surface temperature changes in the northern coast of the South China Sea. J. Asian Earth Sci. 135, 268–280 (2017).
Fan, Z. et al. Worldwide patterns of genomic variation and admixture in gray wolves. Genome Res. 26, 163–173 (2016).
Wurster, C. M. et al. Forest contraction in north equatorial Southeast Asia during the Last Glacial Period. Proc. Natl Acad. Sci. USA 107, 15508–15511 (2010).
Mellars, P. Going east: new genetic and archaeological perspectives on the modern human colonization of Eurasia. Science 313, 796–800 (2006).
Fumihito, A. et al. One subspecies of the red junglefowl (Gallus gallus gallus) suffices as the matriarchic ancestor of all domestic breeds. Proc. Natl Acad. Sci. USA 91, 12505–12509 (1994).
Alberto, F. J. et al. Convergent genomic signatures of domestication in sheep and goats. Nat. Commun. 9, 813 (2018).
Naval-Sanchez, M. et al. Sheep genome functional annotation reveals proximal regulatory elements contributed to the evolution of modern breeds. Nat. Commun. 9, 859 (2018).
Wang, G. D. et al. The genomics of selection in dogs and the parallel evolution between dogs and humans. Nat. Commun. 4, 1860 (2013).
Daly, K. G. et al. Ancient goat genomes reveal mosaic domestication in the Fertile Crescent. Science 361, 85–88 (2018).
Schubert, M. et al. Prehistoric genomes reveal the genetic foundation and cost of horse domestication. Proc. Natl Acad. Sci. USA 111, E5661–E5669 (2014).
Kong, Y. Btrim: a fast, lightweight adapter and quality trimming program for next-generation sequencing technologies. Genomics 98, 152–153 (2011).
Li, H. Toward better understanding of artifacts in variant calling from high-coverage samples. Bioinformatics 30, 2843–2851 (2014).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 26, 1641–1650 (2009).
Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88, 76–82 (2011).
Patterson, N., Price, A. L. & Reich, D. Population structure and eigenanalysis. PLoS Genet. 2, e190 (2006).
Kopelman, N. M., Mayzel, J., Jakobsson, M., Rosenberg, N. A. & Mayrose, I. Clumpak: a program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 15, 1179–1191 (2015).
Chang, C. C. et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015).
Gutenkunst, R. N., Hernandez, R. D., Williamson, S. H. & Bustamante, C. D. Inferring the joint demographic history of multiple populations from multidimensional SNP frequency data. PLoS Genet. 5, e1000695 (2009).
Reich, D., Thangaraj, K., Patterson, N., Price, A. L. & Singh, L. Reconstructing Indian population history. Nature 461, 489–494 (2009).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
McLaren, W. et al. The Ensembl Variant Effect Predictor. Genome Biol. 17, 122 (2016).
Reimand, J., Arak, T. & Vilo, J. g:Profiler-a web server for functional interpretation of gene lists (2011 update). Nucleic Acids Res. 39, W307–W315 (2011).
Acknowledgements
We thank Andrew Lawler for his comments on the manuscript, and Wenming Zhao, Lili Dong, and Bixia Tang for their help with constructing chicken genomic variant database. This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (CAS, XDA2004010301), the National Natural Science Foundation of China (31621062 to Y.-P.Z., 31771415 and 31801054 to M.-S.W., U1902204 and 31822048 to D.-D.W., 31771405 to M.-S.P., and 31822052 to Y.J.), the Yunnan Provincial Science and Technology Department grant to Y.-P.Z. and D.-D.W., the European Research Council grant (ERC-2013-StG-337574-UNDEAD to G.L.), the Arts and Humanities Research Council (AH/L006979/1 to G.L.), the CAS-President’s International Fellowship Initiative Award (2016VBB049 to M.T., 2018VBC0016 to L.A.F.F., 2016VBA050 to A.E., and 2016VBC051 to N.Y.H.) and the West Light Foundation of CAS (Y902401081 to M.-S.W.). C.S. also thanks the support of Unit of Excellence 2019 on Biodiversity and Natural Resources Management, University of Phayao (UoE62005). The Youth Innovation Promotion Association of CAS also provided support to M.-S.W., D.-D.W. and M.-S.P. N.O.O. also thanks the CAS-TWAS President’s Fellowship Program for Doctoral Candidates. Animal Branch of the Germplasm Bank of Wild Species of CAS (the Large Research Infrastructure Funding) also supported this project. The Chinese Government contribution to CAAS-ILRI Joint Laboratory on Livestock and Forage Genetic Resources in Beijing (2018-GJHZ-01) is appreciated. This publication has been prepared within the framework of the UNEP/GEF project “Development and application of decision-support tools to conserve and sustainably use genetic diversity in indigenous livestock and wild relatives”, and it contributes to the CGIAR Research Program on Livestock. This work was also supported by the Animal Branch of the Germplasm Bank of Wild Species, CAS (the Large Research Infrastructure Funding). Due to the limit on the number of corresponding authors, Dr. Greger Larson cannot be listed as co-corresponding author, although his contribution deserves.
Author information
Authors and Affiliations
Contributions
Y.-P.Z., D.-D.W., J.-L.H., M.-S.W., M.-S.P. and Y.J. conceived the project and designed the research. M.-S.W., L.A.F.F., M.-S.P. and M.L. performed most of the analysis with contributions from S.W., N.O.O., S.-S.D., C.M., H.-M.C., Q.-K.S., Y.-H.L., L.Z., J.-F.S., X.G., Z.-Q.Z. and S.-H.F. J.-J. Z., Y.-J.W., M.-S.W., X.-Y.Y., M.-L.L, and M.-M.Y. conducted the wetlab experiments. X.-M.L., X.-Z.J., Q.-H.N. and S.J.L. contributed genome sequencing data. J.-L.H., A.E., C.S., N.Y.H., H.A., S. Suladari, M.S.A.Z., S.K., S. Sohrabi, H.K.-K., E.L., S.C., H.G.T.N.G., T.M.S., H.Z., J.-Q.J., M.-L.L., H.-M.C., C.M., S.-S.D., A.K.F.H. B., M.S.K., G.L.L.P.S, T.-T. L., O.A.M. and M.N.M.I. did sample collection and prepared the DNA samples. M.T. provided and coordinated genome sequencing of Indian samples in India. M.-S.W., L.A.F.F., G.L., A.E., M.-S.P. and J.-L.H. drafted the manuscript with inputs from all authors. G.L., L.A.F.F., J.P., B.S., J.-L.H., M.S., Y.-P.Z., D.-D.W., M.-S.W., G.Z., M.-S.P., M.T., O.H. and A.E. revised the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, MS., Thakur, M., Peng, MS. et al. 863 genomes reveal the origin and domestication of chicken. Cell Res 30, 693–701 (2020). https://doi.org/10.1038/s41422-020-0349-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41422-020-0349-y
This article is cited by
-
Population structure, selection signal and introgression of gamecocks revealed by whole genome sequencing
Journal of Animal Science and Biotechnology (2025)
-
Genetic traceability, conservation effectiveness, and selection signatures analysis based on ancestral information: a case study of Beijing-You chicken
BMC Genomics (2025)
-
Genome-environment association analysis reveals climate-driven adaptation of chickens
Genetics Selection Evolution (2025)
-
Bayesian analyses of radiocarbon dates suggest multiple origins of ceramic technology in Early Holocene Africa
Nature Communications (2025)
-
Genome-wide local ancestry and the functional consequences of admixture in African and European cattle populations
Heredity (2025)