Abstract
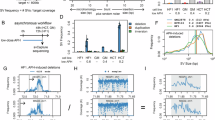
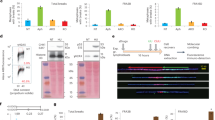
Common fragile sites (CFSs) are genomic loci prone to the formation of breaks or gaps on metaphase chromosomes. They are hotspots for chromosome rearrangements and structural variations, which have been extensively implicated in carcinogenesis, aging, and other pathological processes. Although many CFSs were identified decades ago, a consensus is still lacking for why they are particularly unstable and sensitive to replication perturbations. This is in part due to the lack of high-resolution mapping data for the vast majority of the CFSs, which has hindered mechanistic interrogations. Here, we seek to map human CFSs with high resolution on a genome-wide scale by sequencing the sites of mitotic DNA synthesis (MiDASeq) that are specific for CFSs. We generated a nucleotide-resolution atlas of MiDAS sites (MDSs) that covered most of the known CFSs, and comprehensively analyzed their sequence characteristics and genomic features. Our data on MDSs tallied well with long-standing hypotheses to explain CFS fragility while highlighting the contributions of late replication timing and large transcription units. Notably, the MDSs also encompassed most of the recurrent double-strand break clusters previously identified in mouse neural stem/progenitor cells, thus bridging evolutionarily conserved break points across species. Moreover, MiDAseq provides an important resource that can stimulate future research on CFSs to further unravel the mechanisms and biological relevance underlying these labile genomic regions.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The data have been deposited in the NCBI Gene Expression Omnibus under accession number GSE149376.
References
Glover, T. W., Wilson, T. E. & Arlt, M. F. Fragile sites in cancer: more than meets the eye. Nat. Rev. Cancer 17, 489–501 (2017).
Glover, T. W., Berger, C., Coyle, J. & Echo, B. DNA polymerase? inhibition by aphidicolin induces gaps and breaks at common fragile sites in human chromosomes. Hum. Genet. 67, 136–142 (1984).
Glover, T. W., Arlt, M. F., Casper, A. M. & Durkin, S. G. Mechanisms of common fragile site instability. Hum. Mol. Genet. 14 Spec No. 2, R197–R205 (2005).
Ruiz-Herrera, A. et al. Conservation of aphidicolin-induced fragile sites in Papionini (Primates) species and humans. Chromosome Res. 12, 683–690 (2004).
Stone, D. M., Jacky, P. B., Hancock, D. D. & Prieur, D. J. Animal model: chromosomal fragile site expression in dogs: I. Breed specific differences. Am. J. Med. Genet. 40, 214–222 (1991).
Matsuyama, A. et al. Fragile site orthologs FHIT/FRA3B and Fhit/Fra14A2: evolutionarily conserved but highly recombinogenic. Proc. Natl. Acad. Sci. USA 100, 14988–14993 (2003).
Helmrich, A., Stout-Weider, K., Hermann, K., Schrock, E. & Heiden, T. Common fragile sites are conserved features of human and mouse chromosomes and relate to large active genes. Genome Res. 16, 1222–1230 (2006).
Pentzold, C. et al. FANCD2 binding identifies conserved fragile sites at large transcribed genes in avian cells. Nucleic Acids Res. 46, 1280–1294 (2018).
Casper, A. M., Nghiem, P., Arlt, M. F. & Glover, T. W. ATR regulates fragile site stability. Cell 111, 779–789 (2002).
Durkin, S. G. & Glover, T. W. Chromosome fragile sites. Annu. Rev. Genet. 41, 169–192 (2007).
Durkin, S. G., Arlt, M. F., Howlett, N. G. & Glover, T. W. Depletion of CHK1, but not CHK2, induces chromosomal instability and breaks at common fragile sites. Oncogene 25, 4381–4388 (2006).
Ozeri-Galai, E., Schwartz, M., Rahat, A. & Kerem, B. Interplay between ATM and ATR in the regulation of common fragile site stability. Oncogene 27, 2109–2117 (2008).
Madireddy, A. et al. FANCD2 facilitates replication through common fragile sites. Mol. Cell 64, 388–404 (2016).
Wang, H. et al. The concerted roles of FANCM and Rad52 in the protection of common fragile sites. Nat. Commun. 9, 2791 (2018).
Özer, Ö. & Hickson, I. D. Pathways for maintenance of telomeres and common fragile sites during DNA replication stress. Open Biol. 8, 180018 (2018).
Okamoto, Y. et al. Replication stress induces accumulation of FANCD2 at central region of large fragile genes. Nucleic Acids Res. 46, 2932–2944 (2018).
Chan, K. L., Palmai-Pallag, T., Ying, S. & Hickson, I. D. Replication stress induces sister-chromatid bridging at fragile site loci in mitosis. Nat. Cell Biol. 11, 753–760 (2009).
Ying, S. et al. MUS81 promotes common fragile site expression. Nat. Cell Biol. 15, 1001–1007 (2013).
Minocherhomji, S. et al. Replication stress activates DNA repair synthesis in mitosis. Nature 528, 286–290 (2015).
Gao, G. et al. Common fragile sites (CFS) and extremely large CFS genes are targets for human papillomavirus integrations and chromosome rearrangements in oropharyngeal squamous cell carcinoma. Genes Chromosomes Cancer 56, 59–74 (2016).
Walline, H. M. et al. Integration of high-risk human papillomavirus into cellular cancer-related genes in head and neck cancer cell lines. Head Neck 39, 840–852 (2017).
Wentzensen, N., Vinokurova, S. & Doeberitz, Mv. K. Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer Res. 64, 3878–3884 (2004).
Feichtinger, W. & Schmid, M. Increased frequencies of sister chromatid exchanges at common fragile sites (1)(q42) and (19)(q13). Hum. Genet. 83, 145–147 (1989).
Glover, T. W. & Stein, C. K. Induction of sister chromatid exchanges at common fragile sites. Am. J. Hum. Genet. 41, 882–890 (1987).
Waisertreiger, I., Popovich, K., Block, M., Anderson, K. R. & Barlow, J. H. Visualizing locus-specific sister chromatid exchange reveals differential patterns of replication stress-induced fragile site breakage. Oncogene 39, 1260–1272 (2020).
Wang, H. et al. BLM prevents instability of structure-forming DNA sequences at common fragile sites. PLoS Genet. 14, e1007816 (2018).
Zheglo, D. et al. The FRA14B common fragile site maps to a region prone to somatic and germline rearrangements within the large GPHN gene. Genes Chromosomes Cancer 58, 284–294 (2018).
Wilson, T. E. et al. Large transcription units unify copy number variants and common fragile sites arising under replication stress. Genome Res. 25, 189–200 (2015).
Zack, T. I. et al. Pan-cancer patterns of somatic copy number alteration. Nat. Genet. 45, 1134–1140 (2013).
Chang, R. et al. Loss of Wwox drives metastasis in triple-negative breast cancer by JAK2/STAT3 axis. Nat. Commun. 9, 3486 (2018).
Mare, S. D. et al. WWOX and p53 dysregulation synergize to drive the development of osteosarcoma. Cancer Res. 76, 6107–6117 (2016).
Wu, D. W. et al. FHIT loss confers cisplatin resistance in lung cancer via the AKT/NF-κB/Slug-mediated PUMA reduction. Oncogene 34, 2505–2515 (2014).
Lee, J. I. et al. Loss of Fhit expression is a predictor of poor outcome in tongue cancer. Cancer Res. 61, 837–841 (2001).
Voutsinos, V., Munk, S. H. N. & Oestergaard, V. H. Common chromosomal fragile sites—conserved failure stories. Genes 9, 580 (2018).
Smith, D. I., Zhu, Y., McAvoy, S. & Kuhn, R. Common fragile sites, extremely large genes, neural development and cancer. Cancer Lett. 232, 48–57 (2006).
Kumar, R. et al. HumCFS: a database of fragile sites in human chromosomes. BMC Genom. 19, 985 (2019).
Wei, P.-C. et al. Long neural genes harbor recurrent DNA break clusters in neural stem/progenitor cells. Cell 164, 644–655 (2016).
Wilhelm, K. et al. Fragile sites as drivers of gene and genome. Evol. Curr. Genet. Med. Rep. 6, 136–143 (2018).
Zhang, H. & Freudenreich, C. H. An AT-rich sequence in human common fragile site FRA16D causes fork stalling and chromosome breakage in S. cerevisiae. Mol. Cell 27, 367–379 (2007).
Zlotorynski, E. Poly(dA:dT) make it and break it. Nat. Rev. Mol. Cell Biol. 19, 619–619 (2018).
Zlotorynski, E. et al. Molecular basis for expression of common and rare fragile sites. Mol. Cell. Biol. 23, 7143–7151 (2003).
Irony-Tur Sinai, M. et al. AT-dinucleotide rich sequences drive fragile site formation. Nucleic Acids Res. 47, 9685–9695 (2019).
Palumbo, E., Matricardi, L., Tosoni, E., Bensimon, A. & Russo, A. Replication dynamics at common fragile site FRA6E. Chromosoma 119, 575–587 (2010).
Hellman, A. et al. Replication delay along FRA7H, a common fragile site on human chromosome 7, leads to chromosomal instability. Mol. Cell. Biol. 20, 4420–4427 (2000).
Tallec, B. L. et al. Molecular profiling of common fragile sites in human fibroblasts. Nat. Struct. Mol. Biol. 18, 1421–1423 (2011).
Letessier, A. et al. Cell-type-specific replication initiation programs set fragility of the FRA3B fragile site. Nature 470, 120–123 (2011).
Palakodeti, A. et al. Impaired replication dynamics at the FRA3B common fragile site. Hum. Mol. Genet. 19, 99–110 (2010).
Kelly, T. & Callegari, A. J. Dynamics of DNA replication in a eukaryotic cell. Proc. Natl. Acad. Sci. USA 116, 4973–4982 (2019).
Lõoke, M. et al. Relicensing of transcriptionally inactivated replication origins in budding yeast. J. Biol. Chem. 285, 40004–40011 (2010).
Helmrich, A., Ballarino, M. & Tora, L. Collisions between replication and transcription complexes cause common fragile site instability at the longest human genes. Mol. Cell 44, 966–977 (2011).
Fungtammasan, A., Walsh, E., Chiaromonte, F., Eckert, K. A. & Makova, K. D. A genome-wide analysis of common fragile sites: what features determine chromosomal instability in the human genome? Genome Res. 22, 993–1005 (2012).
Bhowmick, R., Minocherhomji, S. & Hickson, I. D. RAD52 facilitates mitotic DNA synthesis following replication stress. Mol. Cell 64, 1117–1126 (2016).
Tubbs, A. et al. Dual roles of Poly(dA:dT) tracts in replication initiation and Fork Collapse. Cell 174, 1127–1142 (2018).
Palozola, K. C. et al. Mitotic transcription and waves of gene reactivation during mitotic exit. Science 358, 119–122 (2017).
Becker, N. A., Thorland, E. C., Denison, S. R., Phillips, L. A. & Smith, D. I. Evidence that instability within the FRA3B region extends four megabases. Oncogene 21, 8713–8722 (2002).
Veerappa, A. M. et al. Global patterns of large copy number variations in the human genome reveal complexity in chromosome organization. Genet. Res. 97, e18 (2015).
Redon, R. et al. Global variation in copy number in the human genome. Nature 444, 444–454 (2006).
Larkin, D. M. et al. Breakpoint regions and homologous synteny blocks in chromosomes have different evolutionary histories. Genome Res. 19, 770–777 (2009).
Vitae, G., Remita, A. M. & Diallo, A. B. Revisiting the landscape of evolutionary breakpoints across human genome using multi-way comparison. bioRxiv https://doi.org/10.1101/696245 (2019).
Woo, Y. H. & Li, W. H. DNA replication timing and selection shape the landscape of nucleotide variation in cancer genomes. Nat. Commun. 3, 1004 (2012).
Geoffrey, F. et al. Formation of chromosomal domains by loop extrusion. Cell Rep. 15, 2038–2049 (2016).
Suhas, S. P. R. et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680 (2014).
Jacqueline, H. B. et al. Identification of early replicating fragile sites that contribute to genome instability. Cell 152, 620–632 (2013).
Mirkin, E. V. & Mirkin, S. M. Replication fork stalling at natural impediments. Microbiol. Mol. Biol. Rev. 71, 13–35 (2007).
Zentner, G. E. & Henikoff, S. Regulation of nucleosome dynamics by histone modifications. Nat. Struct. Mol. Biol. 20, 259–266 (2013).
Venkatesh, S. & Workman, J. L. Histone exchange, chromatin structure and the regulation of transcription. Nat. Rev. Mol. Cell Biol. 16, 178–189 (2015).
Bell, O., Tiwari, V. K., Thomä, N. H. & Schübeler, D. Determinants and dynamics of genome accessibility. Nat. Rev. Genet. 12, 554–564 (2011).
Richards, E. J. & Elgin, S. C. R. Epigenetic codes for heterochromatin formation and silencing: Rounding up the usual suspects. Cell 108, 489–500 (2002).
Easwaran, H. et al. A DNA hypermethylation module for the stem/progenitor cell signature of cancer. Genome Res. 22, 837–849 (2012).
Ibarra, A., Benner, C., Tyagi, S., Cool, J. & Hetzer, M. W. Nucleoporin-mediated regulation of cell identity genes. Genes Dev. 30, 2253–2258 (2016).
Farnham P, S. M., Cayting P. Histone modifications by ChIP-seq from ENCODE/Stanford/Yale/USC/Harvard https://genome.ucsc.edu/cgi-bin/hgTrackUi?db=hg19&g=wgEncodeSydhHistone (2011).
Matthew, G. G., Stuart, S. L., Laurie, A. B., Rudolf, J. & Richard, A. Y. A chromatin landmark and transcription initiation at most promoters in human cells. Cell 130, 77–88 (2007).
Tianyi, Z., Sarah, C. & Neil, B. The interplay of histone modifications—writers that read. EMBO Rep. 16, 1467–1481 (2015).
Pelliccia, F., Bosco, N., Curatolo, A. & Rocchi, A. Replication timing of two human common fragile sites: FRA1H and FRA2G. Cytogenet. Genome Res. 121, 196–200 (2008).
Sarni, D., et al. Replication timing and transcription identifies a novel fragility signature under replication stress. bioRxiv https://doi.org/10.1101/716951 (2019).
Brison, O. et al. Transcription-mediated organization of the replication initiation program across large genes sets common fragile sites genome-wide. Nat. Commun. 10, 5693 (2019).
Moiseeva, T. N. et al. An ATR and CHK1 kinase signaling mechanism that limits origin firing during unperturbed DNA replication. Proc. Natl. Acad. Sci. USA 116, 13374–13383 (2019).
Wang, Y. et al. Temporal association of ORCA/LRWD1 to late-firing origins during G1 dictates heterochromatin replication and organization. Nucleic Acids Res. 45, 2490–2502 (2017).
Xiong, W., Li, J., Zhang, E. & Huang, H. BMAL1 regulates transcription initiation and activates circadian clock gene expression in mammals. Biochem. Biophys. Res. Commun. 473, 1019–1025 (2016).
Paulsen, M. T. et al. Use of Bru-Seq and BruChase-Seq for genome-wide assessment of the synthesis and stability of RNA. Methods 67, 45–54 (2014).
Canela, A. et al. DNA breaks and end resection measured genome-wide by end sequencing. Mol. Cell 63, 898–911 (2016).
Crosetto, N. et al. Nucleotide-resolution DNA double-strand break mapping by next-generation sequencing. Nat. Methods 10, 361–365 (2013).
Hosseini, S. A. et al. Common chromosome fragile sites in human and murine epithelial cells and FHIT/FRA3B loss-induced global genome instability. Genes Chromosomes Cancer 52, 1017–1029 (2013).
Probst, A. V., Dunleavy, E. & Almouzni, G. Epigenetic inheritance during the cell cycle. Nat. Rev. Mol. Cell Biol. 10, 192–206 (2009).
Sarah, M. R. et al. Design of a synthetic yeast genome. Science 355, 1040–1044 (2017).
A fully designed yeast genome. Nat. Methods 14, 469–469, https://doi.org/10.1038/nmeth.4272 (2017).
Macheret, M. et al. High-resolution mapping of mitotic DNA synthesis regions and common fragile sites in the human genome through direct sequencing. Cell Res. https://doi.org/10.1038/s41422-020-0358-x (2020).
Marchal, C. et al. Genome-wide analysis of replication timing by next-generation sequencing with E/L Repli-seq. Nat. Protoc. 13, 819–839 (2018).
Mori, H., Evans-Yamamoto, D., Ishiguro, S., Tomita, M. & Yachie, N. Fast and global detection of periodic sequence repeats in large genomic resources. Nucleic Acids Res. 47, e8 (2019).
Acknowledgements
This work was supported by grants to Songmin Ying from the Ministry of Science and Technology of China (2016YFA0100301), the National Natural Science Foundation of China (81870007, 81920108001), the Zhejiang Provincial Natural Science Foundation (LD19H160001), and the Zhejiang Provincial Program for the Cultivation of High‐Level Innovative Health Talents (2016‐63). We thank Prof. Ian Hickson (University of Copenhagen, Denmark) for advice on the analysis of MiDAS, and Dr. Jianming Zeng (University of Macau) for valuable suggestions on bioinformatics. We deeply thank Prof. Cheng Li (Peking University) for the courtesy of data on TAD. Thanks for the technical support by the Core Facilities, Zhejiang University School of Medicine.
Author information
Authors and Affiliations
Contributions
F. Ji and H.L. designed the experiments. F. Ji, H.L., F.Z., X.G., S.L., M.Z.S. and H.C. conducted the experiments. S.P., L.O., F. Jia and Z.F. performed bioinformatics analysis. H.L. and F. Ji wrote the manuscript. X.W. and T.L. generated and analyzed the Hi-C data. W.L. and Z.C. analyzed, interpreted the data. S.Y. and H.S. initiated the study, instructed the project and interpreted the data. All authors edited the manuscript and proofread it.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Ji, F., Liao, H., Pan, S. et al. Genome-wide high-resolution mapping of mitotic DNA synthesis sites and common fragile sites by direct sequencing. Cell Res 30, 1009–1023 (2020). https://doi.org/10.1038/s41422-020-0357-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41422-020-0357-y
This article is cited by
-
DNA double-strand break end resection factors and WRN facilitate mitotic DNA synthesis in human cells
Nature Communications (2025)
-
Replication stress induces POLQ-mediated structural variant formation throughout common fragile sites after entry into mitosis
Nature Communications (2024)
-
RECQL4 is not critical for firing of human DNA replication origins
Scientific Reports (2024)
-
FANCD2 genome binding is nonrandom and is enriched at large transcriptionally active neural genes prone to copy number variation
Functional & Integrative Genomics (2024)
-
Mitotic DNA synthesis in response to replication stress requires the sequential action of DNA polymerases zeta and delta in human cells
Nature Communications (2023)