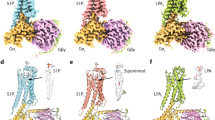
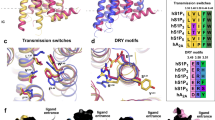
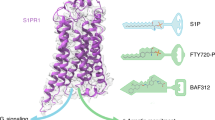
Abstract
Sphingosine-1-phosphate (S1P) is an important bioactive lipid molecule in cell membrane metabolism and binds to G protein-coupled S1P receptors (S1PRs) to regulate embryonic development, physiological homeostasis, and pathogenic processes in various organs. S1PRs are lipid-sensing receptors and are therapeutic targets for drug development, including potential treatment of COVID-19. Herein, we present five cryo-electron microscopy structures of S1PRs bound to diverse drug agonists and the heterotrimeric Gi protein. Our structural and functional assays demonstrate the different binding modes of chemically distinct agonists of S1PRs, reveal the mechanical switch that activates these receptors, and provide a framework for understanding ligand selectivity and G protein coupling.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
All data produced or analyzed in this study are included in the main text or the Supplementary Materials. The cryo-EM density maps and atomic coordinates have been deposited in the Electron Microscopy Data Bank (EMDB) and Protein Data Bank (PDB) under accession numbers EMD-31341 and 7EVY for the siponimod–S1PR1 complex; EMD-31342 and 7EVZ for cenerimod–S1PR1 complex; EMD-31343 and 7EW0 for the ozanimod–S1PR1 complex; EMD-31349 and 7EW7 for the SEW2871–S1PR1 complex; and EMD-31344 and 7EW1 for the siponimod–S1PR5 complex.
References
Obinata, H. & Hla, T. Sphingosine 1-phosphate and inflammation. Int. Immunol. 31, 617–625 (2019).
Proia, R. L. & Hla, T. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J. Clin. Invest. 125, 1379–1387 (2015).
Rosen, H., Stevens, R. C., Hanson, M., Roberts, E. & Oldstone, M. B. Sphingosine-1-phosphate and its receptors: structure, signaling, and influence. Annu. Rev. Biochem. 82, 637–662 (2013).
Jozefczuk, E., Guzik, T. J. & Siedlinski, M. Significance of sphingosine-1-phosphate in cardiovascular physiology and pathology. Pharmacol. Res. 156, 104793 (2020).
Kihara, Y., Maceyka, M., Spiegel, S. & Chun, J. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br. J. Pharmacol. 171, 3575–3594 (2014).
Hannun, Y. A. & Obeid, L. M. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9, 139–150 (2008).
Mendelson, K., Zygmunt, T., Torres-Vázquez, J., Evans, T. & Hla, T. Sphingosine 1-phosphate receptor signaling regulates proper embryonic vascular patterning. J. Biol. Chem. 288, 2143–2156 (2013).
Mendelson, K., Evans, T. & Hla, T. Sphingosine 1-phosphate signalling. Development 141, 5–9 (2014).
Pelletier, D. & Hafler, D. A. Fingolimod for multiple sclerosis. N. Engl. J. Med. 366, 339–347 (2012).
Pyne, N. J. & Pyne, S. Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer 10, 489–503 (2010).
Abu-Farha, M. et al. The role of lipid metabolism in COVID-19 virus infection and as a drug target. Int. J. Mol. Sci. 21, 3544 (2020).
Dyckman, A. J. Modulators of sphingosine-1-phosphate pathway biology: recent advances of sphingosine-1-phosphate receptor 1 (S1P1) agonists and future perspectives. J. Med. Chem. 60, 5267–5289 (2017).
Strader, C. R., Pearce, C. J. & Oberlies, N. H. Fingolimod (FTY720): a recently approved multiple sclerosis drug based on a fungal secondary metabolite. J. Nat. Prod. 74, 900–907 (2011).
Wacker, D., Stevens, R. C. & Roth, B. L. How ligands illuminate GPCR molecular pharmacology. Cell 170, 414–427 (2017).
Al-Salama, Z. T. Siponimod: first global approval. Drugs 79, 1009–1015 (2019).
Lamb, Y. N. Ozanimod: first approval. Drugs 80, 841–848 (2020).
Scott, F. L. et al. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity. Br. J. Pharmacol. 173, 1778–1792 (2016).
McGowan, E. M., Haddadi, N., Nassif, N. T. & Lin, Y. Targeting the SphK-S1P-SIPR pathway as a potential therapeutic approach for COVID-19. Int. J. Mol. Sci. 21, 7189 (2020).
Hanson, M. A. et al. Crystal structure of a lipid G protein-coupled receptor. Science 335, 851–855 (2012).
Koehl, A. et al. Structure of the micro-opioid receptor-Gi protein complex. Nature 558, 547–552 (2018).
Kohno, T. & Igarashi, Y. Roles for N-glycosylation in the dynamics of Edg-1/S1P1 in sphingosine 1-phosphate-stimulated cells. Glycoconj. J. 21, 497–501 (2004).
Chrencik, J. E. et al. Crystal structure of antagonist bound human lysophosphatidic acid receptor 1. Cell 161, 1633–1643 (2015).
Krishna Kumar, K. et al. Structure of a signaling cannabinoid receptor 1-G protein complex. Cell 176, 448–458.e12 (2019).
Pan, S. et al. Discovery of BAF312 (Siponimod), a potent and selective S1P receptor modulator. ACS Med. Chem. Lett. 4, 333–337 (2013).
Venkatakrishnan, A. J. et al. Diverse GPCRs exhibit conserved water networks for stabilization and activation. Proc. Natl. Acad. Sci. USA 116, 3288–3293 (2019).
Mobbs, J. I. et al. Structures of the human cholecystokinin 1 (CCK1) receptor bound to Gs and Gq mimetic proteins provide insight into mechanisms of G protein selectivity. PLoS Biol. 19, e3001295 (2021).
Cartier, A. & Hla, T. Sphingosine 1-phosphate: Lipid signaling in pathology and therapy. Science 366, eaar5551 (2019).
Sanna, M. G. et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J. Biol. Chem. 279, 13839–13848 (2004).
Flemming, S. et al. Sphingosine-1-phosphate receptor-1 agonist Sew2871 causes severe cardiac side effects and does not improve microvascular barrier breakdown in sepsis. Shock 49, 71–81 (2018).
Marsolais, D. et al. Local not systemic modulation of dendritic cell S1P receptors in lung blunts virus-specific immune responses to influenza. Mol. Pharmacol. 74, 896–903 (2008).
Fujiwara, Y. et al. Identification of the hydrophobic ligand binding pocket of the S1P1 receptor. J. Biol. Chem. 282, 2374–2385 (2007).
Mizuno, H. & Kihara, Y. Druggable lipid GPCRs: past, present, and prospects. Adv. Exp. Med. Biol. 1274, 223–258 (2020).
Zhao, C. et al. Structural insights into sphingosine-1-phosphate recognition and ligand selectivity of S1PR3-Gi signaling complexes. Cell Res. https://doi.org/10.1038/s41422-021-00567-w (2021).
McAllister, S. D. et al. Structural mimicry in class A G protein-coupled receptor rotamer toggle switches: the importance of the F3.36(201)/W6.48(357) interaction in cannabinoid CB1 receptor activation. J. Biol. Chem. 279, 48024–48037 (2004).
Israeli, H. et al. Structure reveals the activation mechanism of the MC4 receptor to initiate satiation signaling. Science 372, 808–814 (2021).
Manglik, A. & Kruse, A. C. Structural basis for G protein-coupled receptor activation. Biochemistry 56, 5628–5634 (2017).
Hua, T. et al. Activation and signaling mechanism revealed by cannabinoid receptor-Gi complex structures. Cell 180, 655–665.e18 (2020).
Xing, C. et al. Cryo-EM structure of the human cannabinoid receptor CB2-Gi signaling complex. Cell 180, 645–654.e13 (2020).
Venkatakrishnan, A. J. et al. Molecular signatures of G-protein-coupled receptors. Nature 494, 185–194 (2013).
Gavel, Y., Steppuhn, J., Herrmann, R. & von Heijne, G. The ‘positive-inside rule’ applies to thylakoid membrane proteins. FEBS Lett. 282, 41–46 (1991).
Xu, P. et al. Structures of the human dopamine D3 receptor-Gi complexes. Mol. Cell 81, 1147–1159.e4 (2021).
Flock, T. et al. Selectivity determinants of GPCR-G-protein binding. Nature 545, 317–322 (2017).
Shao, Z. et al. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature 540, 602–606 (2016).
Xu, X. et al. Binding pathway determines norepinephrine selectivity for the human beta1AR over beta2AR. Cell Res. 31, 569–579 (2021).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
Scheres, S. H. W. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Pettersen, E. F. et al. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Waterhouse, A. et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 (2018).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221 (2010).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D. Biol. Crystallogr. 66, 12–21 (2010).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Xiao, P. et al. Ligand recognition and allosteric regulation of DRD1-Gs signaling complexes. Cell 184, 943–956.e18 (2021).
Ping, Y. Q. et al. Structures of the glucocorticoid-bound adhesion receptor GPR97-Go complex. Nature 589, 620–626 (2021).
Acknowledgements
Cryo-EM data were collected at SKLB West China Cryo-EM Center in Sichuan University and Cryo-EM Center in Southern University of Science and Technology (SUSTech), processed at SKLB Duyu High Performance Computing Center in Sichuan University. This work was supported by Sichuan University start-up funding (20822041D4057 to Z. Su), the Natural Science Foundation of China grants (82041016 and 32070049 to Z. Su); Ministry of Science and Technology of China grant (2019YFA0508800 to Z. Shao), Science and Technology department of Sichuan Province (2020YJ0208 to Z. Shao).
Author information
Authors and Affiliations
Contributions
Z. Shao, W.Y. and C.W. designed the cellular assays and analyzed results. Y.Y., W.W. and C.W. designed the expression constructs, purified the S1PRS–Gi–scFv16 complex, prepared the final samples for data collection toward the structures, and participated in figure and paper preparation with assistance from Q.L., Z.L., K.L. and S.Y.; Z. Su designed the cryo-EM experiments; G.J. prepared the cryo-EM grids, collected and processed cryo-EM data under the supervision of Z. Su; Y.Y., L.C. and G.J. built and refined the models under the supervision of Z. Shao and Z. Su; Z. Shao supervised the overall project, and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Yuan, Y., Jia, G., Wu, C. et al. Structures of signaling complexes of lipid receptors S1PR1 and S1PR5 reveal mechanisms of activation and drug recognition. Cell Res 31, 1263–1274 (2021). https://doi.org/10.1038/s41422-021-00566-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41422-021-00566-x
This article is cited by
-
S1PR3-driven positive feedback loop sustains STAT3 activation and keratinocyte hyperproliferation in psoriasis
Cell Death & Disease (2025)
-
S1PR1-biased activation drives the resolution of endothelial dysfunction-associated inflammatory diseases by maintaining endothelial integrity
Nature Communications (2025)
-
Irilone and Lupinisoflavone C as Potential Plant-Based Modulators of S1PR1 for Neuroimmune Modulation in Multiple Sclerosis: Insights from Molecular Docking and Dynamics
Journal of Molecular Neuroscience (2025)
-
Structural mechanisms of potent lysophosphatidic acid receptor 1 activation by nonlipid basic agonists
Communications Biology (2024)
-
Structural basis for lysophosphatidylserine recognition by GPR34
Nature Communications (2024)