Abstract
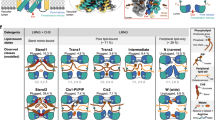
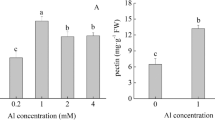
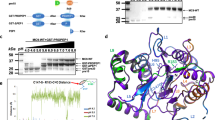
The plant aluminum (Al)-activated malate transporter ALMT1 mediates the efflux of malate to chelate the Al in acidic soils and underlies the plant Al resistance. Here we present cryo-electron microscopy (cryo-EM) structures of Arabidopsis thaliana ALMT1 (AtALMT1) in the apo, malate-bound, and Al-bound states at neutral and/or acidic pH at up to 3.0 Å resolution. The AtALMT1 dimer assembles an anion channel and each subunit contains six transmembrane helices (TMs) and six cytosolic α-helices. Two pairs of Arg residues are located in the center of the channel pore and contribute to malate recognition. Al binds at the extracellular side of AtALMT1 and induces conformational changes of the TM1–2 loop and the TM5–6 loop, resulting in the opening of the extracellular gate. These structures, along with electrophysiological measurements, molecular dynamic simulations, and mutagenesis study in Arabidopsis, elucidate the structural basis for Al-activated malate transport by ALMT1.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The cryo-EM density maps and coordinates of ALMT1apo/pH5, ALMT1apo/pH7.5, ALMT1malate/pH7.5, ALMT1Al/pH5, and ALMT1M60A/Al/pH5 have been deposited in the Electron Microscopy Data Bank (EMDB) under accession numbers EMD-32084, EMD-32085, EMD-32086, EMD-32087, and EMD-32050, and in the RCSB Protein Data Bank (PDB) under accession codes 7VQ3, 7VQ4, 7VQ5, 7VQ7, and 7VOJ.
References
von Uexküll, H. R. & Mutert, E. Global extent, development and economic impact of acid soils. Plant Soil. 171, 1–15 (1995).
Kochian, L. V. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 237–260 (1995).
Ryan, P. R., Delhaize, E. & Jones, D. L. Function and mechanism of organic anion exudation from plant roots. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 527–560 (2001).
Kochian, L. V., Piñeros, M. A., Liu, J. & Magalhaes, J. V. Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 66, 571–598 (2015).
Ma, J. F., Chen, Z. C. & Shen, R. F. Molecular mechanisms of Al tolerance in gramineous plants. Plant Soil. 381, 1–12 (2014).
Sasaki, T. et al. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 37, 645–653 (2004).
Hoekenga, O. A. et al. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 103, 9738–9743 (2006).
Ligaba, A., Katsuhara, M., Ryan, P. R., Shibasaka, M. & Matsumoto, H. The BnALMT1 and BnALMT2 genes from rape encode aluminum-activated malate transporters that enhance the aluminum resistance of plant cells. Plant Physiol. 142, 1294–1303 (2006).
Liang, C. et al. Low pH, aluminum, and phosphorus coordinately regulate malate exudation through GmALMT1 to improve soybean adaptation to acid soils. Plant Physiol. 161, 1347–1361 (2013).
Fontecha, G. et al. Candidate gene identification of an aluminum-activated organic acid transporter gene at the Alt4 locus for aluminum tolerance in rye (Secale cereale L.). Theor. Appl. Genet. 114, 249–260 (2006).
Sharma, T., Dreyer, I., Kochian, L. & Pineros, M. A. The ALMT family of organic acid transporters in plants and their involvement in detoxification and nutrient security. Front Plant Sci. 7, 1488 (2016).
Zhang, X., Long, Y., Huang, J. & Xia, J. Molecular mechanisms for coping with Al toxicity in plants. Int. J. Mol. Sci. 20, 1551 (2019).
Ding, Z. J., Yan, J. Y., Xu, X. Y., Li, G. X. & Zheng, S. J. WRKY46 functions as a transcriptional repressor of ALMT1, regulating aluminum-induced malate secretion in Arabidopsis. Plant J. 76, 825–835 (2013).
Iuchi, S. et al. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc. Natl. Acad. Sci. USA 104, 9900–9905 (2007).
Furuichi, T. et al. An extracellular hydrophilic carboxy-terminal domain regulates the activity of TaALMT1, the aluminum-activated malate transport protein of wheat. Plant J. 64, 47–55 (2010).
Ligaba, A. et al. Functional, structural and phylogenetic analysis of domains underlying the Al sensitivity of the aluminum-activated malate/anion transporter, TaALMT1. Plant J. 76, 766–780 (2013).
Ligaba, A., Maron, L., Shaff, J. O. N., Kochian, L. & PiÑEros, M. Maize ZmALMT2 is a root anion transporter that mediates constitutive root malate efflux. Plant Cell Environ. 35, 1185–1200 (2012).
Piñeros, M. A. et al. Not all ALMT1-type transporters mediate aluminum-activated organic acid responses: the case of ZmALMT1 - an anion-selective transporter. Plant J. 53, 352–367 (2007).
De Angeli, A., Zhang, J., Meyer, S. & Martinoia, E. AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat. Commun. 4, 1804 (2013).
Kovermann, P. et al. The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant J. 52, 1169–1180 (2007).
Meyer, S. et al. AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J. 63, 1054–1062 (2010).
Meyer, S. et al. Malate transport by the vacuolar AtALMT6 channel in guard cells is subject to multiple regulation. Plant J. 67, 247–257 (2011).
Sasaki, T. et al. Closing plant stomata requires a homolog of an aluminum-activated malate transporter. Plant Cell Physiol. 51, 354–365 (2010).
De Angeli, A. et al. The vacuolar channel VvALMT9 mediates malate and tartrate accumulation in berries of Vitis vinifera. Planta 238, 283–291 (2013).
Xu, M. et al. The barley anion channel, HvALMT1, has multiple roles in guard cell physiology and grain metabolism. Physiol. Plantarum 153, 183–193 (2015).
Palmer, A. J., Baker, A. & Muench, S. P. The varied functions of aluminium-activated malate transporters-much more than aluminium resistance. Biochem. Soc. Trans. 44, 856–862 (2016).
Ramesh, S. A. et al. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat. Commun. 6, 7879 (2015).
Deneka, D., Sawicka, M., Lam, A. K. M., Paulino, C. & Dutzler, R. Structure of a volume-regulated anion channel of the LRRC8 family. Nature 558, 254–259 (2018).
Laverty, D. et al. Cryo-EM structure of the human alpha1beta3gamma2 GABAA receptor in a lipid bilayer. Nature 565, 516–520 (2019).
Park, E., Campbell, E. B. & MacKinnon, R. Structure of a CLC chloride ion channel by cryo-electron microscopy. Nature 541, 500–505 (2017).
Paulino, C., Kalienkova, V., Lam, A. K. M., Neldner, Y. & Dutzler, R. Activation mechanism of the calcium-activated chloride channel TMEM16A revealed by cryo-EM. Nature 552, 421–425 (2017).
Dang, S. et al. Cryo-EM structures of the TMEM16A calcium-activated chloride channel. Nature 552, 426–429 (2017).
Zhang, W. H. et al. Characterization of the TaALMT1 protein as an Al3+-activated anion channel in transformed tobacco (Nicotiana tabacum L.) cells. Plant Cell Physiol. 49, 1316–1330 (2008).
Delhaize, E. et al. Engineering high-level aluminum tolerance in barley with the ALMT1 gene. Proc. Natl. Acad. Sci. USA 101, 15249–15254 (2004).
Morales-Perez, C. L., Noviello, C. M. & Hibbs, R. E. Manipulation of subunit stoichiometry in heteromeric membrane proteins. Structure 24, 797–805 (2016).
Goehring, A. et al. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nat. Protoc. 9, 2574–2585 (2014).
Li, X. et al. Molecular basis for ligand activation of the human KCNQ2 channel. Cell Res. 31, 52–61 (2020).
Liu, S., Chang, S., Han, B., Xu, L. & Guo, J. Cryo-EM structures of the human cation-chloride cotransporter KCC1. Science 366, 505–508 (2019).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: Real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Scheres, S. H. W. RELION: Implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2009).
Schrodinger, L. The PyMOL molecular graphics system. Version 1, 0 (2015).
Pettersen, E. F. et al. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Zhang, Y. et al. F-box protein RAE1 regulates the stability of the aluminum-resistance transcription factor STOP1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 116, 319–327 (2019).
Delhaize, E., Ryan, P. R. & Randall, P. J. Aluminum tolerance in wheat (Triticum aestivum L.) II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol. 103, 695–702 (1993).
Luo, Y. H., Yu, X. F., Ma, C., Yang, F. & Yang, W. Effects of calcium-binding sites in the S2-S3 loop on human and Nematostella vectensis TRPM2 channel gating processes. J. Zhejiang Univ. Sci. B 20, 972–982 (2019).
Yu, P. et al. Direct Gating of the TRPM2 Channel by cADPR via Specific Interactions with the ADPR Binding Pocket. Cell Rep. 27, 3684–3695 (2019). e3684.
Yuhuan, L., Xiafei, Y., Cheng, M., Jianhong, L. & Wei, Y. Identification of a Novel EF-Loop in the N-terminus of TRPM2 Channel Involved in Calcium Sensitivity. Front. Pharmacol. 9, 581 (2018).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2016).
Vanommeslaeghe, K. et al. CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 31, 671–690 (2009).
Yesselman, J. D., Price, D. J., Knight, J. L. & Brooks, C. L. MATCH: An atom-typing toolset for molecular mechanics force fields. J. Comput. Chem. 33, 189–202 (2012).
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Acknowledgements
Single-particle cryo-EM data were collected at the Center of Cryo-Electron Microscopy at Zhejiang University. We thank Dr. Xing Zhang and Dr. Shenghai Chang for support in facility access and data acquisition. We thank Dr. Chao Feng Huang for kindly providing the almt1 mutant seeds. We thank Dr. Nam Nguyen for his assistance in the paper preparation. This work was supported in part by the Ministry of Science and Technology (2020YFA0908501 and 2018YFA0508100 to J.G., 2016YFA0501100 to X.Z.), the National Natural Science Foundation of China (31870724 to J.G., 82030108, 31872796 and 81571127 to W.Y., 31730006 to S.J.Z., and 31600606 to X.Z.), National Major Special Project on New Drug Innovation of China (2018ZX09711001-004-005 to W.Y.), Zhejiang Provincial Natural Science Foundation (LR19C050002 to J.G., LR16H090001 to W.Y.), and the Fundamental Research Funds for the Central Universities (2021FZZX001-28 to J.G. and S.J.Z.). J.G. is supported by MOE Frontier Science Center for Brain Science & Brain-Machine Integration, Zhejiang University. X.Z. is supported by Guangdong Provincial Key Laboratory of Brain Connectome and Behavior (2017B030301017) and CAS Key Laboratory of Brain Connectome and Manipulation (2019DP173024).
Author information
Authors and Affiliations
Contributions
J.G., W.Y., and S.J.Z. conceived and supervised the project. J.W., X.Z., Y.X., and X.L. prepared the samples, performed data acquisition, structure determination, and data analysis. X.Y. and Y.L. performed electrophysiological studies. Z.J.D. and T.Y. performed the in vivo mutagenesis studies. X.X. performed molecular dynamics simulation. All authors participated in the data analysis and paper preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, J., Yu, X., Ding, Z.J. et al. Structural basis of ALMT1-mediated aluminum resistance in Arabidopsis. Cell Res 32, 89–98 (2022). https://doi.org/10.1038/s41422-021-00587-6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41422-021-00587-6
This article is cited by
-
Structural basis for malate-driven, pore lipid-regulated activation of the Arabidopsis vacuolar anion channel ALMT9
Nature Communications (2025)
-
Dynamics of Avena sativa L. Roots Under Phosphorus and Iron Variation
Journal of Soil Science and Plant Nutrition (2025)
-
GmAP2 enhances plant tolerance to aluminum toxicity and phosphorus deficiency in Arabidopsis
Plant Cell Reports (2025)
-
Sensing the toxic aluminum cations in acidic soils
Cell Research (2024)
-
The LRR receptor-like kinase ALR1 is a plant aluminum ion sensor
Cell Research (2024)