Abstract
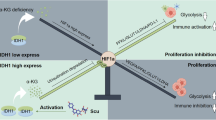
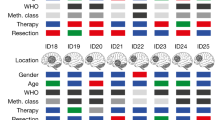
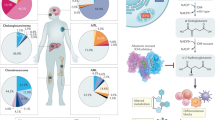
Mutant isocitrate dehydrogenase 1 (mIDH1) drives tumorigenesis via producing oncometabolite R-2-hydroxyglutarate (R-2-HG) across various tumor types. However, mIDH1 inhibitors appear only effective in hematological tumors. The therapeutic benefit in solid tumors remains elusive, likely due to the complex tumor microenvironment. In this study, we discover that R-2-HG produced by IDH1-mutant tumor cells is preferentially imported into vascular endothelial cells and remodels mitochondrial respiration to promote tumor angiogenesis, conferring a therapeutic vulnerability in IDH1-mutant solid tumors. Mechanistically, SLC1A1, a Na+-dependent glutamate transporter that is preferentially expressed in endothelial cells, facilitates the influx of R-2-HG from the tumor microenvironment into the endothelial cells as well as the intracellular trafficking of R-2-HG from cytoplasm to mitochondria. R-2-HG hijacks SLC1A1 to promote mitochondrial Na+/Ca2+ exchange, which activates the mitochondrial respiratory chain and fuels vascular endothelial cell migration in tumor angiogenesis. SLC1A1 deficiency in mice abolishes mIDH1-promoted tumor angiogenesis as well as the therapeutic benefit of mIDH1 inhibitor in solid tumors. Moreover, we report that HH2301, a newly discovered mIDH1 inhibitor, shows promising efficacy in treating IDH1-mutant cholangiocarcinoma in preclinical models. Together, we identify a new role of SLC1A1 as a gatekeeper of R-2-HG-mediated crosstalk between IDH1-mutant tumor cells and vascular endothelial cells, and demonstrate the therapeutic potential of mIDH1 inhibitors in treating IDH1-mutant solid tumors via disrupting R-2-HG-promoted tumor angiogenesis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Mardis, E. R. et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med. 361, 1058–1066 (2009).
Parsons, D. W. et al. An integrated genomic analysis of human glioblastoma multiforme. Science 321, 1807–1812 (2008).
Yan, H. et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 360, 765–773 (2009).
Borger, D. R. et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist 17, 72–79 (2012).
DiNardo, C. D. et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N. Engl. J. Med. 378, 2386–2398 (2018).
Tateishi, K. et al. Extreme vulnerability of IDH1 mutant cancers to NAD+ depletion. Cancer Cell 28, 773–784 (2015).
Waitkus, M. S. & Yan, H. Targeting isocitrate dehydrogenase mutations in cancer: emerging evidence and diverging strategies. Clin. Cancer Res. 27, 383–388 (2021).
Moure, C. J. et al. CRISPR editing of mutant IDH1 R132H induces a CpG methylation-low state in patient-derived glioma models of G-CIMP. Mol. Cancer Res. 17, 2042–2050 (2019).
Mellinghoff, I. K. et al. Ivosidenib in isocitrate dehydrogenase 1-mutated advanced glioma. J Clin Oncol. 38, 3398–3406 (2020).
Abou-Alfa, G. K. et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 21, 796–807 (2020).
Dang, L. et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462, 739–744 (2009).
Lu, C. et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483, 474–478 (2012).
Xu, W. et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 19, 17–30 (2011).
M Gagné, L., Boulay, K., Topisirovic, I., Huot, M. E. & Mallette, F. A. Oncogenic activities of IDH1/2 mutations: from epigenetics to cellular signaling. Trends Cell Biol. 27, 738–752 (2017).
Friedrich, M. et al. Tryptophan metabolism drives dynamic immunosuppressive myeloid states in IDH-mutant gliomas. Nat. Cancer 2, 723–740 (2021).
Bunse, L. et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat. Med. 24, 1192–1203 (2018).
Rohle, D. et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 340, 626–630 (2013).
Folkman, J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285, 1182–1186 (1971).
Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 29, 15–18 (2002).
Seok, J., Yoon, S. H., Lee, S. H., Jung, J. H. & Lee, Y. M. The oncometabolite d2hydroxyglutarate induces angiogenic activity through the vascular endothelial growth factor receptor 2 signaling pathway. Int. J. Oncol. 54, 753–763 (2019).
Potente, M., Gerhardt, H. & Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887 (2011).
Gentile, M. T., Pastorino, O., Bifulco, M. & Colucci-D’Amato, L. HUVEC tube-formation assay to evaluate the impact of natural products on angiogenesis. J. Vis. Exp. https://doi.org/10.3791/58591 (2019).
Ye, J. L. et al. EAAT3 promotes amino acid transport and proliferation of porcine intestinal epithelial cells. Oncotarget 7, 38681–38692 (2016).
Ximerakis, M. et al. Single-cell transcriptomic profiling of the aging mouse brain. Nat. Neurosci. 22, 1696–1708 (2019).
Yu, X. et al. Cryo-EM structures of the human glutamine transporter SLC1A5 (ASCT2) in the outward-facing conformation. Elife 8, e48120 (2019).
Garaeva, A. A. et al. Cryo-EM structure of the human neutral amino acid transporter ASCT2. Nat. Struct. Mol. Biol. 25, 515–521 (2018).
Heinzelmann, G. & Kuyucak, S. Molecular dynamics simulations of the mammalian glutamate transporter EAAT3. PLoS ONE 9, e92089 (2014).
Jain, R. K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005).
Mancuso, M. R. et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J. Clin. Invest. 116, 2610–2621 (2006).
Zhao, S. et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science 324, 261–265 (2009).
Fu, X. et al. 2-Hydroxyglutarate Inhibits ATP Synthase and mTOR Signaling. Cell Metab. 22, 508–515 (2015).
Qing, Y. et al. R-2-hydroxyglutarate attenuates aerobic glycolysis in leukemia by targeting the FTO/m(6)A/PFKP/LDHB axis. Mol Cell. 81, 922–939.e9 (2021).
Achouri, Y. et al. Identification of a dehydrogenase acting on D-2-hydroxyglutarate. Biochem. J. 381, 35–42 (2004).
Magi, S. et al. Physical and functional interaction of NCX1 and EAAC1 transporters leading to glutamate-enhanced ATP production in brain mitochondria. PLoS ONE 7, e34015 (2012).
Magi, S. et al. Glutamate-induced ATP synthesis: relationship between plasma membrane Na+/Ca2+ exchanger and excitatory amino acid transporters in brain and heart cell models. Mol. Pharmacol. 84, 603–614 (2013).
Piccirillo, S., Castaldo, P., Macri, M. L., Amoroso, S. & Magi, S. Glutamate as a potential “survival factor” in an in vitro model of neuronal hypoxia/reoxygenation injury: leading role of the Na(+)/Ca(2+) exchanger. Cell Death Dis. 9, 731 (2018).
Xiong, J. et al. Rapid affinity purification of intracellular organelles using a twin strep tag. J. Cell Sci. 132, jcs235390 (2019).
Pellerin, L. How astrocytes feed hungry neurons. Mol. Neurobiol. 32, 59–72 (2005).
Castaldo, P. et al. Role of the mitochondrial sodium/calcium exchanger in neuronal physiology and in the pathogenesis of neurological diseases. Prog. Neurobiol. 87, 58–79 (2009).
Minelli, A. et al. Cellular and subcellular localization of Na+-Ca2+ exchanger protein isoforms, NCX1, NCX2, and NCX3 in cerebral cortex and hippocampus of adult rat. Cell Calcium 41, 221–234 (2007).
Gobbi, P. et al. Mitochondrial localization of Na+/Ca2+ exchangers NCX1-3 in neurons and astrocytes of adult rat brain in situ. Pharmacol. Res. 56, 556–565 (2007).
Rizzuto, R., De Stefani, D., Raffaello, A. & Mammucari, C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 13, 566–578 (2012).
De Bock, K., Georgiadou, M. & Carmeliet, P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 18, 634–647 (2013).
Pollard, T. D. & Borisy, G. G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 (2003).
De Bock, K. et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154, 651–663 (2013).
Li, S. et al. Transient assembly of F-actin on the outer mitochondrial membrane contributes to mitochondrial fission. J. Cell Biol. 208, 109–123 (2015).
Lei, J. et al. Compound having mutant idh inhibitory activity, preparation method and use thereof, National Center for Biotechnology Information. PubChem Patent Summary for WO-2017162133-A1. https://pubchem.ncbi.nlm.nih.gov/patent/WO-2017162133-A1.
Saha, S. K. et al. Isocitrate dehydrogenase mutations confer dasatinib hypersensitivity and SRC dependence in intrahepatic cholangiocarcinoma. Cancer Discov. 6, 727–739 (2016).
Choi, S. et al. Vandetanib inhibits growth of adenoid cystic carcinoma in an orthotopic nude mouse model. Clin. Cancer Res. 14, 5081–5089 (2008).
Leyva-Illades, D., McMillin, M., Quinn, M. & Demorrow, S. Cholangiocarcinoma pathogenesis: Role of the tumor microenvironment. Transl. Gastrointest. Cancer 1, 71–80 (2012).
Sirica, A. E. & Gores, G. J. Desmoplastic stroma and cholangiocarcinoma: clinical implications and therapeutic targeting. Hepatology 59, 2397–2402 (2014).
Peraldo-Neia, C. et al. Transcriptomic analysis and mutational status of IDH1 in paired primary-recurrent intrahepatic cholangiocarcinoma. BMC Genomics 19, 440 (2018).
Wang, P. et al. Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene 32, 3091–3100 (2013).
Saha, S. K., Parachoniak, C. A. & Bardeesy, N. IDH mutations in liver cell plasticity and biliary cancer. Cell Cycle 13, 3176–3182 (2014).
Saha, S. K. et al. Mutant IDH inhibits HNF-4alpha to block hepatocyte differentiation and promote biliary cancer. Nature 513, 110–114 (2014).
Dong, L. et al. Proteogenomic characterization identifies clinically relevant subgroups of intrahepatic cholangiocarcinoma. Cancer Cell 40, 70–87.e15 (2022).
Carbonneau, M. et al. The oncometabolite 2-hydroxyglutarate activates the mTOR signalling pathway. Nat. Commun. 7, 12700 (2016).
Su, R. et al. R-2HG exhibits anti-tumor activity by targeting FTO/m(6)A/MYC/CEBPA signaling. Cell 172, 90–105.e23 (2018).
Li, T. et al. D-2-Hydroxyglutarate is necessary and sufficient for isocitrate dehydrogenase 1 mutant-induced MIR148A promoter methylation. Mol. Cancer Res. 16, 947–960 (2018).
Kim, G. H. et al. IDH1(R132H) causes resistance to HDAC inhibitors by increasing NANOG in glioblastoma cells. Int. J. Mol. Sci. 20, 2679 (2019).
Chowdhury, R. et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 12, 463–469 (2011).
Bailey, C. G. et al. Loss-of-function mutations in the glutamate transporter SLC1A1 cause human dicarboxylic aminoaciduria. J. Clin. Invest. 121, 446–453 (2011).
Muhlhausen, C. et al. Membrane translocation of glutaric acid and its derivatives. J. Inherit. Metab. Dis. 31, 188–193 (2008).
Jin, N. et al. Identification of metabolic vulnerabilities of receptor tyrosine kinases-driven cancer. Nat. Commun. 10, 2701 (2019).
Boudker, O., Ryan, R. M., Yernool, D., Shimamoto, K. & Gouaux, E. Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature 445, 387–393 (2007).
Acknowledgements
We thank Prof. Xin Pan (National Center of Biomedical Analysis, Beijing, China) for kindly providing the 4mt-GCaMP6 and 4mt-ATeam1.03 plasmids and for the valuable advice for the study. We thank Prof. Guangwei Du (McGovern Medical School, The University of Texas Health Science Center at Houston, Houston, USA) for providing the Mito-2Strep plasmid, Prof. Qiang Gao (Zhongshan Hospital, Fudan University, Shanghai, China) for gifting the primary cholangiocarcinoma cells, Prof. Lei Chen (Second Military Medical University, Shanghai, China) for gifting LICCF cholangiocarcinoma cell line and Prof. Lili Ji (Shanghai University of Traditional Chinese Medicine, Shanghai, China) for gifting primary HRECs. This work was supported by the National Natural Science Foundation of China (81821005, 91957126, 81903640, 91957120), and the Program of Shanghai Academic Research Leader (20XD1424800).
Author information
Authors and Affiliations
Contributions
M.H. conceived the project and supervised the study; Z.C. initiated the project and discovered R-2-HG-promoted endothelial cell migration and mitochondrial activation; N.A. contributed to the early stage of SLC1A1 discovery; X.W. and J.X. established the role of SLC1A1 in R-2-HG uptake, discovered SLC1A1-dependent mitochondrial Na+/Ca2+ exchange, and demonstrated the role of SLC1A1 in R-2-HG-promoted angiogenesis in vivo; S.T. characterized mIDH1 inhibitors; L.J. invented HH2301; Y.Zhang and L.L. supervised the preclinical development of HH2301 and verified HH2301 compound-related information; T.W., S.Cao., Y.Liu., A.B., Yuehong.C., X.G., Y.D. and Y.Zheng. performed part of experiments; Y.F., Y.Li., L.Y. and L.T. provided technique assistance; Qingli.Z., X.L. and J.L. conducted R-2-HG measurement; S.Chen, S.G., K.M., M.W. and C.L. purified IDH1 proteins and performed MST assay; S.-H.L. performed metabolome analysis; S.Z. and Qiansen.Z. did SLC1A1 molecular modeling and docking; Y.S. and Yi.C. performed part of PDX studies; L.Z. and M.T. performed proteomics analysis; X.W. and J.X. prepared all figures; M.H., X.W. and J.X. wrote the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.J. was a full-time employee of Haihe Biopharma Co, Ltd..Y.Zhang and L.L. are full-time employees of Haihe Biopharma Co, Ltd.. Haihe Biopharma has filed a patent for HH2301 and L.J., M.H., S.T. and X.L. are co-inventors. M.H. is a paid consultant of Haihe Biopharma.
Rights and permissions
About this article
Cite this article
Wang, X., Chen, Z., Xu, J. et al. SLC1A1-mediated cellular and mitochondrial influx of R-2-hydroxyglutarate in vascular endothelial cells promotes tumor angiogenesis in IDH1-mutant solid tumors. Cell Res 32, 638–658 (2022). https://doi.org/10.1038/s41422-022-00650-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41422-022-00650-w
This article is cited by
-
Targeting mitochondrial homeostasis as a cancer treatment strategy: current status and future prospects
Molecular Cancer (2026)
-
Amino acid metabolic reprogramming: future prospects for cholangiocarcinoma therapy
Cell Death Discovery (2026)
-
Bridging epigenomics and tumor immunometabolism: molecular mechanisms and therapeutic implications
Molecular Cancer (2025)
-
TCA cycle-derived oncometabolites in cancer and the immune microenvironment
Journal of Biomedical Science (2025)
-
Mitochondrial metabolism and cancer therapeutic innovation
Signal Transduction and Targeted Therapy (2025)