Abstract
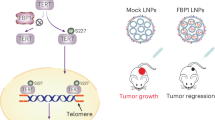
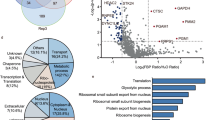
Emerging evidence demonstrates that some metabolic enzymes that phosphorylate soluble metabolites can also phosphorylate a variety of protein substrates as protein kinases to regulate cell cycle, apoptosis and many other fundamental cellular processes. However, whether a metabolic enzyme dephosphorylates protein as a protein phosphatase remains unknown. Here we reveal the gluconeogenic enzyme fructose 1,6-biphosphatase 1 (FBP1) that catalyzes the hydrolysis of fructose 1,6-bisphosphate (F-1,6-BP) to fructose 6-phosphate (F-6-P) as a protein phosphatase by performing a high-throughput screening of metabolic phosphatases with molecular docking followed by molecular dynamics (MD) simulations. Moreover, we identify IκBα as the substrate of FBP1-mediated dephosphorylation by performing phosphoproteomic analysis. Mechanistically, FBP1 directly interacts with and dephosphorylates the serine (S) 32/36 of IκBα upon TNFα stimulation, thereby inhibiting NF-κB activation. MD simulations indicate that the catalytic mechanism of FBP1-mediated IκBα dephosphorylation is similar to F-1,6-BP dephosphorylation, except for higher energetic barriers for IκBα dephosphorylation. Functionally, FBP1-dependent NF-κB inactivation suppresses colorectal tumorigenesis by sensitizing tumor cells to inflammatory stresses and preventing the mobilization of myeloid-derived suppressor cells. Our finding reveals a previously unrecognized role of FBP1 as a protein phosphatase and establishes the critical role of FBP1-mediated IκBα dephosphorylation in colorectal tumorigenesis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lu, Z. & Hunter, T. Metabolic kinases moonlighting as protein kinases. Trends Biochem. Sci. 43, 301–310 (2018).
Li, B. et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature 513, 251–255 (2014).
Li, F. et al. FBP1 loss disrupts liver metabolism and promotes tumorigenesis through a hepatic stellate cell senescence secretome. Nat. Cell Biol. 22, 728–739 (2020).
Dong, C. et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell 23, 316–331 (2013).
Balkwill, F. & Mantovani, A. Inflammation and cancer: back to Virchow? Lancet 357, 539–545 (2001).
Feagins, L. A., Souza, R. F. & Spechler, S. J. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 6, 297–305 (2009).
Janakiram, N. B. & Rao, C. V. The role of inflammation in colon cancer. Adv. Exp. Med. Biol. 816, 25–52 (2014).
Baker, K. J., Houston, A. & Brint, E. IL-1 Family members in cancer; two sides to every story. Front. Immunol. 10, 1197 (2019).
Taniguchi, K. & Karin, M. NF-kappaB, inflammation, immunity and cancer: coming of age. Nat. Rev. Immunol. 18, 309–324 (2018).
Hayden, M. S. & Ghosh, S. Shared principles in NF-kappaB signaling. Cell 132, 344–362 (2008).
Hayden, M. S. & Ghosh, S. Regulation of NF-kappaB by TNF family cytokines. Semin. Immunol. 26, 253–266 (2014).
Pierce, B. G., Hourai, Y. & Weng, Z. Accelerating protein docking in ZDOCK using an advanced 3D convolution library. PLoS ONE 6, e24657 (2011).
Steitz, T. A. & Steitz, J. A. A general two-metal-ion mechanism for catalytic RNA. Proc. Natl. Acad. Sci. USA 90, 6498–6502 (1993).
Long, A. G., Lundsmith, E. T. & Hamilton, K. E. Inflammation and colorectal cancer. Curr. Colorectal. Cancer Rep. 13, 341–351 (2017).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Chavez-Galan, L., Arenas-Del Angel, M. C., Zenteno, E., Chavez, R. & Lascurain, R. Cell death mechanisms induced by cytotoxic lymphocytes. Cell. Mol. Immunol. 6, 15–25 (2009).
Rath, P. C. & Aggarwal, B. B. TNF-induced signaling in apoptosis. J. Clin. Immunol. 19, 350–364 (1999).
Van Antwerp, D. J., Martin, S. J., Verma, I. M. & Green, D. R. Inhibition of TNF-induced apoptosis by NF-kappa B. Trends Cell Biol. 8, 107–111 (1998).
Patel, M., Horgan, P. G., McMillan, D. C. & Edwards, J. NF-kappaB pathways in the development and progression of colorectal cancer. Transl. Res. 197, 43–56 (2018).
Sun, S. C. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat. Rev. Immunol. 17, 545–558 (2017).
Li, Q. & Verma, I. M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2, 725–734 (2002).
Youn, J. I., Nagaraj, S., Collazo, M. & Gabrilovich, D. I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 181, 5791–5802 (2008).
Tavazoie, M. F. et al. LXR/ApoE activation restricts innate immune suppression in cancer. Cell 172, 825–840.e18 (2018).
Kumar, V., Patel, S., Tcyganov, E. & Gabrilovich, D. I. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 37, 208–220 (2016).
Li, K. et al. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal. Transduct. Target. Ther. 6, 362 (2021).
Hirata, H. et al. Decreased expression of fructose-1,6-bisphosphatase associates with glucose metabolism and tumor progression in hepatocellular carcinoma. Cancer Res. 76, 3265–3276 (2016).
Hunter, R. W. et al. Metformin reduces liver glucose production by inhibition of fructose-1-6-bisphosphatase. Nat. Med. 24, 1395–1406 (2018).
Sun, W. et al. PPM1A and PPM1B act as IKKbeta phosphatases to terminate TNFalpha-induced IKKbeta-NF-kappaB activation. Cell. Signal. 21, 95–102 (2009).
Tsuchiya, Y. et al. Distinct B subunits of PP2A regulate the NF-kappaB signalling pathway through dephosphorylation of IKKbeta, IkappaBalpha and RelA. FEBS Lett. 591, 4083–4094 (2017).
Wei, X. M. et al. Protein tyrosine phosphatase L1 represses endothelial-mesenchymal transition by inhibiting IL-1beta/NF-kappaB/Snail signaling. Acta Pharmacol. Sin. 41, 1102–1110 (2020).
Pons, S. & Torres-Aleman, I. Insulin-like growth factor-I stimulates dephosphorylation of ikappa B through the serine phosphatase calcineurin (protein phosphatase 2B). J. Biol. Chem. 275, 38620–38625 (2000).
Nakai, Y., Irie, S. & Sato, T. A. Identification of IkappaBalpha as a substrate of Fas-associated phosphatase-1. Eur. J. Biochem. 267, 7170–7175 (2000).
Wang, X. et al. UDP-glucose accelerates SNAI1 mRNA decay and impairs lung cancer metastasis. Nature 571, 127–131 (2019).
Wu, Y. B. et al. Concurrent quantification of proteome and phosphoproteome to reveal system-wide association of protein phosphorylation and gene expression. Mol. Cell. Proteom. 8, 2809–2826 (2009).
Gao, Q. et al. Integrated proteogenomic characterization of HBV-related hepatocellular carcinoma. Cell 179, 561–577.e22 (2019).
Wang, S. et al. motifeR: An integrated web software for identification and visualization of protein posttranslational modification motifs. Proteomics 19, e1900245 (2019).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Trott, O. & Olson, A. J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Compu. Chem. 31, 455–461 (2010).
Pan, Y. et al. Ultrafast tracking of a single live virion during the invagination of a cell membrane. Small 11, 2782–2788 (2015).
Stratakis, C. A. & Carney, J. A. The triad of paragangliomas, gastric stromal tumours and pulmonary chondromas (Carney triad), and the dyad of paragangliomas and gastric stromal sarcomas (Carney–Stratakis syndrome): molecular genetics and clinical implications. J. Intern. Med. 266, 43–52 (2009).
Homeyer, N., Horn, A. H., Lanig, H. & Sticht, H. AMBER force-field parameters for phosphorylated amino acids in different protonation states: phosphoserine, phosphothreonine, phosphotyrosine, and phosphohistidine. J. Mol. Model. 12, 281–289 (2006).
Case, D. et al. Amber 2016. San Francisco: University of California, 2016.
Hornak, V. et al. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins 65, 712–725 (2006).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for stimulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Berendsen, H. J. C., Postma, J. P. M., Gunsteren, W. F. V., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984).
Ryckaert, J. P., Ciccotti, G. & Berendsen, H. J. C. Numerical integration of cartesian equatios of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys. 23, 327–341 (1977).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Roe, D. R. & Cheatham, T. E. III PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 9, 3084–3095 (2013).
Liu, P.-S. et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 18, 985–994 (2017).
Proudfoot, M. et al. High throughput screening of purified proteins for enzymatic activity. Methods Mol. Biol. 426, 331–341 (2008).
Kuznetsova, E. et al. Structure and activity of the metal-independent fructose-1,6-bisphosphatase YK23 from Saccharomyces cerevisiae. J. Biol. Chem. 285, 21049–21059 (2010).
Debnath, S. et al. A trapped human PPM1A-phosphopeptide complex reveals structural features critical for regulation of PPM protein phosphatase activity. J. Biol. Chem. 293, 7993–8008 (2018).
Vichai, V. & Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 1, 1112–1116 (2006).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (92253305, 92053203 and 32025013) to W.Y.; the National Key R&D Program of China (2022YFA0806201 and 2019YFA0802000) to W.Y.; CAS Project for Young Scientists in Basic Research (YSBR-014) to W.Y.; Program of Shanghai Academic/Technology Research Leader (20XD1424400) to W.Y.; the Innovative Research Team of High-level Local Universities in Shanghai (SHSMU-ZLCX20212302) to W.Y.; the Strategic Priority Research Program of Chinese Academy of Sciences (XDB 37000000) to G.L.; the National Natural Science Foundation of China (21933010) to G.L.; the National Natural Science Foundation of China (21907094) to H.C.; the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2022265) to Y.Zhang; Shanghai Science and Technology Development Funds (22QA1409900) to Y.Zhang. We gratefully acknowledge the support of the Sanofi Scholarship Program. We also thank all the core facilities of Shanghai Institute of Biochemistry and Cell Biology for technical support.
Author information
Authors and Affiliations
Contributions
W.Y. conceived and designed the study. W.Z., X.W., Y.Zhang, H.Y., and H.Z. performed the experiments. G.L. designed the molecular dynamics strategy. G.L., H.C., and Y.L. performed the simulations and data analysis. T.L. and Q.L. provided reagents and pathological assistance. H.G. assisted in reviewing the paper. Y.Zhao provided constructive suggestions. W.Y. and G.L. wrote the manuscript with comments from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, W., Chu, H., Zhang, Y. et al. Fructose-1,6-bisphosphatase 1 dephosphorylates IκBα and suppresses colorectal tumorigenesis. Cell Res 33, 245–257 (2023). https://doi.org/10.1038/s41422-022-00773-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41422-022-00773-0
This article is cited by
-
The non-metabolic function of 6PGD coordinates CCNA2 and HMGA2 expression to drive colorectal cancer progression and drug response
Journal of Experimental & Clinical Cancer Research (2025)
-
RIPK2 promotes colorectal cancer metastasis by protecting YAP degradation from ITCH-mediated ubiquitination
Cell Death & Disease (2025)
-
Moonlighting functions of glucose metabolic enzymes and metabolites in cancer
Nature Reviews Cancer (2025)
-
Mitochondrial metabolic reprogramming in colorectal cancer: mechanisms of resistance and future clinical interventions
Cell Death Discovery (2025)
-
MLN4924 suppresses tumor metabolism and growth of clear cell renal cell carcinoma by stabilizing nuclear FBP1
Cell Death Discovery (2025)