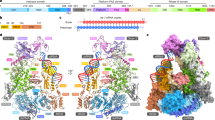
Abstract
In animals, AGO-clade Argonaute proteins utilize small interfering RNAs (siRNAs) as guides to recognize target with complete complementarity, resulting in target RNA cleavage that is a critical step for target silencing. These proteins feature a constricted nucleic acid-binding channel that limits base pairing between the guide and target beyond the seed region. How the AGO–siRNA complexes overcome this structural limitation and achieve efficient target cleavage remains unclear. We performed cryo-electron microscopy of human AGO–siRNA complexes bound to target RNAs of increasing lengths to examine the conformational changes associated with target recognition and cleavage. Initially, conformational transition propagates from the opening of the PAZ domain and extends through a repositioning of the PIWI–L1–N domain toward the binding channel, facilitating the capture of siRNA–target duplex. Subsequent extension of base pairing drives the downward movement of the PIWI–L1–N domain to enable catalytic activation. Finally, further base pairing toward the 3′ end of siRNA destabilizes the PAZ–N domain, resulting in a “uni-lobed” architecture, which might facilitate the multi-turnover action of the AGO–siRNA enzyme complex. In contrast to PIWI-clade Argonautes, the “uni-lobed” structure of the AGO complex makes multiple contacts with the target in the central region of the siRNA–target duplex, positioning it within the catalytic site. Our findings shed light on the stepwise mechanisms by which the AGO–siRNA complex executes target RNA cleavage and offer insights into the distinct operational modalities of AGO and PIWI proteins in achieving such cleavage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Meister, G. Argonaute proteins: Functional insights and emerging roles. Nat. Rev. Genet. 14, 447–459 (2013).
Wang, X., Ramat, A., Simonelig, M. & Liu, M. F. Emerging roles and functional mechanisms of PIWI-interacting RNAs. Nat. Rev. Mol. Cell Biol. 24, 123–141 (2023).
Bartel, D. P. Metazoan microRNAs. Cell 173, 20–51 (2018).
Tang, G. siRNA and miRNA: An insight into RISCs. Trends Biochem. Sci. 30, 106–114 (2005).
Ghildiyal, M., Xu, J., Seitz, H., Weng, Z. & Zamore, P. D. Sorting of Drosophila small silencing RNAs partitions microRNA* strands into the RNA interference pathway. RNA 16, 43–56 (2010).
Ozata, D. M., Gainetdinov, I., Zoch, A., O’Carroll, D. & Zamore, P. D. PIWI-interacting RNAs: small RNAs with big functions. Nat. Rev. Genet. 20, 89–108 (2019).
Lewis, B. P., Burge, C. B. & Bartel, D. P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20 (2005).
Carthew, R. W. & Sontheimer, E. J. Origins and mechanisms of miRNAs and siRNAs. Cell 136, 642–655 (2009).
Eichhorn, S. W. et al. MRNA destabilization is the dominant effect of mammalian microRNAs by the time substantial repression ensues. Mol. Cell 56, 104–115 (2014).
Bazzini, A. A., Lee, M. T. & Giraldez, A. J. Ribosome profiling shows that miR-430 reduces translation before causing mRNA decay in zebrafish. Science 336, 233–237 (2012).
Mathonnet, G. et al. MicroRNA inhibition of translation initiation in vitro by targeting the cap-binding complex eIF4F. Science 317, 1764–1767 (2007).
Djuranovic, S., Nahvi, A. & Green, R. miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science 336, 237–240 (2012).
Haley, B. & Zamore, P. D. Kinetic analysis of the RNAi enzyme complex. Nat. Struct. Mol. Biol. 11, 599–606 (2004).
Chiu, Y. L. & Rana, T. M. RNAi in human cells: Basic structural and functional features of small interfering RNA. Mol. Cell 10, 549–561 (2002).
Chiu, Y. L. & Rana, T. M. siRNA function in RNAi: A chemical modification analysis. RNA 9, 1034–1048 (2003).
Zamore, P. D., Tuschl, T., Sharp, P. A. & Bartel, D. P. RNAi: Double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 101, 25–33 (2000).
Jadhav, V., Vaishnaw, A., Fitzgerald, K. & Maier, M. A. RNA interference in the era of nucleic acid therapeutics. Nat. Biotechnol. 42, 394–405 (2024).
Wilkins, C. et al. RNA interference is an antiviral defence mechanism in Caenorhabditis elegans. Nature 436, 1044–1047 (2005).
Wang, X. H. et al. RNA interference directs innate immunity against viruses in adult Drosophila. Science 312, 452–454 (2006).
Cheloufi, S., Dos Santos, C. O., Chong, M. M. W. & Hannon, G. J. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature 465, 584–589 (2010).
Cifuentes, D. et al. A novel miRNA processing pathway independent of dicer requires argonaute2 catalytic activity. Science 328, 1694–1698 (2010).
Yang, S. et al. Conserved vertebrate mir-451 provides a platform for Dicer-independent, Ago2-mediated microRNA biogenesis. Proc. Natl. Acad. Sci. USA 107, 15163–15168 (2010).
Jee, D. et al. Dual strategies for argonaute2-mediated biogenesis of erythroid miRNAs underlie conserved requirements for slicing in mammals. Mol. Cell 69, 265–278.e6 (2018).
Elkayam, E. et al. The structure of human argonaute-2 in complex with miR-20a. Cell 150, 100–110 (2012).
Schirle, N. T. & MacRae, I. J. The crystal structure of human argonaute2. Science 336, 1037–1040 (2012).
Schirle, N. T., Sheu-Gruttadauria, J. & MacRae, I. J. Structural basis for microRNA targeting. Science 346, 608–613 (2014).
Martinez, J. & Tuschl, T. RISC is a 5′ phosphomonoester-producing RNA endonuclease. Genes Dev. 18, 975–980 (2004).
Wee, L. M., Flores-Jasso, C. F., Salomon, W. E. & Zamore, P. D. Argonaute divides its RNA guide into domains with distinct functions and RNA-binding properties. Cell 151, 1055–1067 (2012).
Becker, W. R. et al. High-throughput analysis reveals rules for target RNA binding and cleavage by AGO2. Mol. Cell 75, 741–755.e11 (2019).
Yuan, Y. R. et al. Crystal structure of A. aeolicus argonaute, a site-specific DNA-guided endoribonuclease, provides insights into RISC-mediated mRNA cleavage. Mol. Cell 19, 405–419 (2005).
Song, J. J., Smith, S. K., Hannon, G. J. & Joshua-Tor, L. Crystal structure of argonaute and its implications for RISC slicer activity. Science 305, 1434–1437 (2004).
Parker, J. S., Roe, S. M. & Barford, D. Crystal structure of a PIWI protein suggests mechanisms for siRNA recognition and slicer activity. EMBO J. 23, 4727–4737 (2004).
Wang, Y. et al. Nucleation, propagation and cleavage of target RNAs in Ago silencing complexes. Nature 461, 754–761 (2009).
Tang, G., Reinhart, B. J., Bartel, D. P. & Zamore, P. D. A biochemical framework for RNA silencing in plants. Genes Dev. 17, 49–63 (2003).
Caudy, A. A. et al. A microRNA in a multiple-turnover RNAi enzyme complex. Science 297, 2056–2060 (2002).
Ameres, S. L., Martinez, J. & Schroeder, R. Molecular basis for target RNA recognition and cleavage by human RISC. Cell 130, 101–112 (2007).
Li, Z. et al. Structural insights into RNA cleavage by PIWI Argonaute. Nature 639, 250–259 (2024).
Schirle, N. T., Sheu-Gruttadauria, J., Chandradoss, S. D., Joo, C. & MacRae, I. J. Water-mediated recognition of t1-adenosine anchors Argonaute2 to microRNA targets. Elife 4, e07646 (2015).
Anzelon, T. A. et al. Structural basis for piRNA targeting. Nature 597, 285–289 (2021).
Wang, Y. et al. Structure of an argonaute silencing complex with a seed-containing guide DNA and target RNA duplex. Nature 456, 921–926 (2008).
Sheng, G. et al. Structure-based cleavage mechanism of Thermus thermophilus argonaute DNA guide strand-mediated DNA target cleavage. Proc. Natl. Acad. Sci. USA 111, 652–657 (2014).
Filipowicz, W. RNAi: The nuts and bolts of the RISC machine. Cell 122, 17–20 (2005).
Rivas, F. V. et al. Purified Argonaute2 and an siRNA form recombinant human RISC. Nat. Struct. Mol. Biol. 12, 340–349 (2005).
De, N. et al. Highly complementary target RNAs promote release of guide RNAs from human argonaute2. Mol. Cell 50, 344–355 (2013).
Hutvágner, G. et al. A cellular function for the RNA-interference enzyme dicer in the maturation of the let-7 small temporal RNA. Science 293, 834–838 (2001).
Van Rooij, E. et al. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 316, 575–579 (2007).
Baccarini, A. et al. Kinetic analysis reveals the fate of a microRNA following target regulation in mammalian cells. Curr. Biol. 21, 369–376 (2011).
Mohamed, A. A., Wang, P. Y., Bartel, D. P. & Vos, S. M. The structural basis for RNA slicing by human Argonaute2. Cell Rep. 44, 115166 (2025).
Sarkar, S., Gebert, L. F. R. & MacRae, I. J. Structural basis for gene silencing by siRNAs in humans. bioRxiv https://doi.org/10.1101/2024.12.05.627081 (2024).
Sheu-Gruttadauria, J. et al. Structural basis for target-directed microRNA degradation. Mol. Cell 75, 1243–1255.e7 (2019).
Wang, P. Y. & Bartel, D. P. The guide-RNA sequence dictates the slicing kinetics and conformational dynamics of the Argonaute silencing complex. Mol. Cell 84, 2918–2934.e11 (2024).
Matsumoto, N. et al. Crystal structure of silkworm PIWI-clade argonaute Siwi bound to piRNA. Cell 167, 484–497.e9 (2016).
Li, Z. et al. Mammalian PIWI–piRNA–target complexes reveal features for broad and efficient target silencing. Nat. Struct. Mol. Biol. 31, 1222–1231 (2024).
Arif, A. et al. GTSF1 accelerates target RNA cleavage by PIWI-clade Argonaute proteins. Nature 608, 618–625 (2022).
Xiol, J. et al. RNA clamping by Vasa assembles a piRNA amplifier complex on transposon transcripts. Cell 157, 1698–1711 (2014).
Dai, S. et al. A family of C. elegans VASA homologs control Argonaute pathway specificity and promote transgenerational silencing. Cell Rep. 40, 111265 (2022).
Flores-Jasso, C. F., Salomon, W. E. & Zamore, P. D. Rapid and specific purification of Argonaute-small RNA complexes from crude cell lysates. RNA 19, 271–279 (2013).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. CryoSPARC: Algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Scheres, S. H. W. RELION: Implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Emsley, P. & Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. Sect. D Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Sect. D Biol. Crystallogr. 66, 213–221 (2010).
Pettersen, E. F. et al. UCSF Chimera — A visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Goddard, T. D. et al. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Acknowledgements
We thank Craig C. Mello, Phillip D. Zamore, Dangsheng Li for insightful suggestions; members of Shen’s lab for discussions; Cryo-EM Facility of Westlake University for providing support on cryo-EM data collection. This work was supported by Westlake Education Foundation, Zhejiang Provincial Foundation of China (2021R01013), the National Natural Science Foundation of China (32070628), Westlake Education Foundation (041010140118), Zhejiang Provincial Key Laboratory Construction Project, and the Westlake Laboratory of Life Sciences to E.Z.S., the National Natural Science Foundation of China (32271261), Zhejiang Provincial Natural Science Foundation of China (LR22C050003), Westlake University (1011103860222B1), and Institutional Startup Grant from the Westlake Education Foundation (101486021901) to J.W.
Author information
Authors and Affiliations
Contributions
E.Z.S. conceived and designed the study. Z.L., Y.Z., Q.X., J.Z., T.Z., J.X. and S.L. performed experiments and analyzed the data. E.Z.S. wrote the manuscript with help from all authors; H.G., Z.Z., J.W. and E.Z.S. reviewed and edited the manuscript. E.Z.S. and J.W. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41422_2025_1114_MOESM14_ESM.mp4
Supplementary Video S1. Overview of cryo-EM density and the structural model of hAGO2D669A-siRNA-target (12-nt). The map and model are colored as in Fig. 1.
41422_2025_1114_MOESM15_ESM.mp4
Supplementary Video S2. Overview of cryo-EM density and the structural model of hAGO2D669A-siRNA-target (14-nt, sesqui-lobed).
41422_2025_1114_MOESM16_ESM.mp4
Supplementary Video S3. Overview of cryo-EM density and the structural model of hAGO2D669A-siRNA-target (14-nt, uni-lobed).
41422_2025_1114_MOESM19_ESM.mp4
Supplementary Video S6. Conformational dynamics of hAGO2 ternary complexes as complementarity extended towards the siRNA 3ʹ end.
41422_2025_1114_MOESM20_ESM.mp4
Supplementary Video S7. Conformational dynamics of MILI ternary complexes as complementarity extended towards the piRNA 3ʹ end.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Z., Xu, Q., Zhang, Y. et al. Mechanistic insights into RNA cleavage by human Argonaute2–siRNA complex. Cell Res 35, 453–464 (2025). https://doi.org/10.1038/s41422-025-01114-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41422-025-01114-7