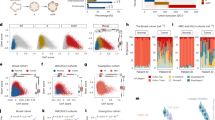
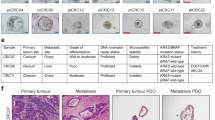
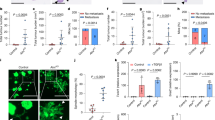
Abstract
Phenotypic plasticity is a hallmark feature driving cancer progression, metastasis, and therapy resistance. Fetal-like transcriptional programs have been increasingly implicated in promoting plastic cell states, yet their roles remain difficult to study due to limitations of existing culture models. Here, we establish a chemically defined patient-derived organoid system that enables long-term expansion of colorectal cancer (CRC) cells while preserving fetal-like features associated with phenotypic plasticity. Using this model, we identify an oncofetal state (OnFS) that is enriched in advanced tumors and linked to key features of plasticity, including epithelial-mesenchymal plasticity, as well as increased metastasis and treatment resistance. Mechanistically, we show that FGF2-AP-1 signaling maintains the OnFS program and associated phenotypic plasticity in CRC. This model offers a powerful platform for studying the fetal-like features underlying cancer cell plasticity and their role in tumor progression and treatment resistance in CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated during the current study are available in the GEO database (GSE261012, GSE261004). All other data used in this study are provided within the article as Source Data or are available from the corresponding authors upon reasonable request.
References
Meacham, C. E. & Morrison, S. J. Tumour heterogeneity and cancer cell plasticity. Nature 501, 328–337 (2013).
Dongre, A. & Weinberg, R. A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 20, 69–84 (2019).
Shibue, T. & Weinberg, R. A. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 14, 611–629 (2017).
Fey, S. K., Vaquero-Siguero, N. & Jackstadt, R. Dark force rising: Reawakening and targeting of fetal-like stem cells in colorectal cancer. Cell Rep. 43, 114270 (2024).
Sharma, A., Bleriot, C., Currenti, J. & Ginhoux, F. Oncofetal reprogramming in tumour development and progression. Nat. Rev. Cancer 22, 593–602 (2022).
Fazilaty, H. & Basler, K. Reactivation of embryonic genetic programs in tissue regeneration and disease. Nat. Genet. 55, 1792–1806 (2023).
Mzoughi, S. et al. Oncofetal reprogramming drives phenotypic plasticity in WNT-dependent colorectal cancer. Nat. Genet. 57, 402–412 (2025).
Nusse, Y. M. et al. Parasitic helminths induce fetal-like reversion in the intestinal stem cell niche. Nature 559, 109–113 (2018).
Moorman, A. R. et al. Progressive plasticity during colorectal cancer metastasis. Nature 637, 947–954 (2024).
Lukonin, I. et al. Phenotypic landscape of intestinal organoid regeneration. Nature 586, 275–280 (2020).
Qu, M. et al. Establishment of intestinal organoid cultures modeling injury-associated epithelial regeneration. Cell Res. 31, 259–271 (2021).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Barkley, D. et al. Cancer cell states recur across tumor types and form specific interactions with the tumor microenvironment. Nat. Genet. 54, 1192–1201 (2022).
Fujii, M. & Sato, T. Somatic cell-derived organoids as prototypes of human epithelial tissues and diseases. Nat. Mater. 20, 156–169 (2021).
Fujii, M. et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell 18, 827–838 (2016).
LeSavage, B. L., Suhar, R. A., Broguiere, N., Lutolf, M. P. & Heilshorn, S. C. Next-generation cancer organoids. Nat. Mater. 21, 143–159 (2021).
van de Wetering, M. et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945 (2015).
Corsini, N. S. & Knoblich, J. A. Human organoids: New strategies and methods for analyzing human development and disease. Cell 185, 2756–2769 (2022).
Wang, J., Sun, S. & Deng, H. Chemical reprogramming for cell fate manipulation: Methods, applications, and perspectives. Cell Stem Cell 30, 1130–1147 (2023).
Xiang, C. et al. Long-term functional maintenance of primary human hepatocytes in vitro. Science 364, 399–402 (2019).
Guan, J. et al. Chemical reprogramming of human somatic cells to pluripotent stem cells. Nature 605, 325–331 (2022).
Vasquez, E. G. et al. Dynamic and adaptive cancer stem cell population admixture in colorectal neoplasia. Cell Stem Cell 29, 1213–1228.e8 (2022).
Qin, X. et al. An oncogenic phenoscape of colonic stem cell polarization. Cell 186, 5554–5568.e18 (2023).
de Lau, W. et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293–297 (2011).
Ying, Q. L. et al. The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 (2008).
Haramis, A. P. et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science 303, 1684–1686 (2004).
He, X. C. et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat. Genet. 36, 1117–1121 (2004).
Koo, J. et al. Live-cell invasive phenotyping uncovers ALK2 as a therapeutic target in LKB1-mutant lung cancer. Cancer Res. 84, 3761–3771 (2024).
Sanvitale, C. E. et al. A new class of small molecule inhibitor of BMP signaling. PLoS One 8, e62721 (2013).
Blay, J. & Brown, K. D. Epidermal growth factor promotes the chemotactic migration of cultured rat intestinal epithelial cells. J. Cell Physiol. 124, 107–112 (1985).
Fujii, M. et al. Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition. Cell Stem Cell 23, 787–793.e6 (2018).
He, G. W. et al. Optimized human intestinal organoid model reveals interleukin-22-dependency of paneth cell formation. Cell Stem Cell 29, 1333–1345.e6 (2022).
Huch, M. et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160, 299–312 (2015).
Grivennikov, S. et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 (2009).
Ye, X. & Weinberg, R. A. Epithelial-mesenchymal plasticity: a central regulator of cancer progression. Trends Cell Biol. 25, 675–686 (2015).
Yang, J. & Weinberg, R. A. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell 14, 818–829 (2008).
Cook, D. P. & Vanderhyden, B. C. Transcriptional census of epithelial-mesenchymal plasticity in cancer. Sci. Adv. 8, eabi7640 (2022).
Mustata, R. C. et al. Identification of Lgr5-independent spheroid-generating progenitors of the mouse fetal intestinal epithelium. Cell Rep. 5, 421–432 (2013).
Bala, P. et al. Aberrant cell state plasticity mediated by developmental reprogramming precedes colorectal cancer initiation. Sci. Adv. 9, eadf0927 (2023).
Gavish, A. et al. Hallmarks of transcriptional intratumour heterogeneity across a thousand tumours. Nature 618, 598–606 (2023).
Nagata, K. et al. Mesothelin expression is correlated with chemoresistance in Stage IV Colorectal Cancer. Ann. Surg. Oncol. 28, 8579–8586 (2021).
Cañellas-Socias, A. et al. Metastatic recurrence in colorectal cancer arises from residual EMP1(+) cells. Nature 611, 603–613 (2022).
Peng, M. et al. The role of Clusterin in cancer metastasis. Cancer Manag. Res. 11, 2405–2414 (2019).
Gregorieff, A., Liu, Y., Inanlou, M. R., Khomchuk, Y. & Wrana, J. L. Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature 526, 715–718 (2015).
Liu-Chittenden, Y. et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 26, 1300–1305 (2012).
Kamimoto, K. et al. Dissecting cell identity via network inference and in silico gene perturbation. Nature 614, 742–751 (2023).
Zanconato, F. et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol. 17, 1218–1227 (2015).
Whiting, F. J. H. & Graham, T. A. Plasticity in metastatic colorectal cancer. Dev. Cell 60, 171–173 (2025).
Moorman, A. et al. Progressive plasticity during colorectal cancer metastasis. Nature 637, 947–954 (2025).
Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141, 1762–1772 (2011).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Wolock, S. L., Lopez, R. & Klein, A. M. Scrublet: computational identification of cell doublets in single-cell transcriptomic data. Cell Syst. 8, 281–291.e9 (2019).
Stuart, T. et al. Comprehensive Integration of Single-Cell Data. Cell 177, 1888–1902.e1821 (2019).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017).
Liu, J. et al. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell 173, 400–416.e11 (2018).
Joanito, I. et al. Single-cell and bulk transcriptome sequencing identifies two epithelial tumor cell states and refines the consensus molecular classification of colorectal cancer. Nat. Genet. 54, 963–975 (2022).
Kotliar, D. et al. Identifying gene expression programs of cell-type identity and cellular activity with singlecell RNA-Seq. Elife 8, e43803 (2019).
He, P. et al. The changing mouse embryo transcriptome at whole tissue and single-cell resolution. Nature 583, 760–767 (2020).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Manders, F. et al. MutationalPatterns: the one stop shop for the analysis of mutational processes. BMC Genomics 23, 134 (2022).
Tirosh, I. et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016).
Leek, J. T., Johnson, W. E., Parker, H. S., Jaffe, A. E. & Storey, J. D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 28, 882–883 (2012).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Acknowledgements
We thank Shicheng Sun, Jingyang Guan, Jinlin Wang for discussion of the manuscript. We thank Hui Wang for technical assistance with High-Content live imaging and 3D reconstruction. We thank the Optical Imaging Core Facility, National Center for Protein Sciences at Peking University in Beijing, China, for assistance with the use of High-Speed Spinning Disk Confocal Microscope, RS 2000, and Ms. Yan Luo for help with the multiplex immunofluorescence assay. We thank the flow cytometry Core at the National Center for Protein Sciences at Peking University, particularly Liying Du, Hongxia Lyu, Huan Yang and Jia Luo for technical help. We thank the National Center for Protein Sciences (Beijing) — the Protein Core at Peking University for assistance with the shRNA library and Dr Tao Xu for technical support. We would like to thank the Department of Laboratory Animals of Peking University Cancer Hospital for technical advice on animal experiments. We thank the High-Performance Computing Platform of the Center for Life Science, Peking University, for the support of bioinformatics calculations. This work was supported by the National Natural Science Foundation of China (32288102) and the National Key R&D Program of China Grant (2022YFA1103103).
Author information
Authors and Affiliations
Contributions
L.X. designed the research, performed experiments, analyzed the data and wrote the manuscript. Y.X., L.W. and C.L. performed the bioinformatics analysis. J.S. performed the PDOX experiments. Z.G., X.W., W.H. and M.L. acquired patient informed consents and clinical samples. Y.W. participated in immunohistochemistry imaging. J.X. wrote and revised the manuscript. H.D. and J.G. designed and supervised the study. M.Q. designed the research, analyzed the data, wrote the manuscript, and supervised the study. All authors edited and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiong, L., Xu, Y., Gao, Z. et al. A patient-derived organoid model captures fetal-like plasticity in colorectal cancer. Cell Res 35, 642–655 (2025). https://doi.org/10.1038/s41422-025-01139-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41422-025-01139-y