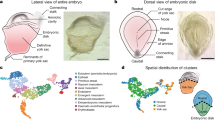
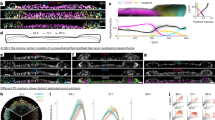
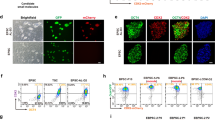
Abstract
Pluripotent stem cells (PSCs) have been derived from various species, but most culture systems stabilize only a single PSC type. By contrast, epiblast cells in vivo exist along a continuum and interact dynamically with both embryonic and extraembryonic cells, interactions missing in standard PSC cultures. This absence limits the self-organizing potential of PSCs and leads to disorganized tissue formation in teratomas. To address this, we developed a unified culture system that supports the stable differentiation of epiblast-like cells into multiple key human gastrulating cell types, collectively called human gastrulating stem cells (hGaSCs). hGaSCs, composed of endoderm-like, mesoderm-like, ectoderm-like, amnion ectoderm-like, and primordial germ cell-like cells, maintain a stable balance during long-term culture. In 3D culture, hGaSCs self-assemble into gastruloid-like structures (hGaSC-gastruloids) that model aspects of a Carnegie Stage 7 human embryo, including gastrulation and germ layer specification. Using hGaSC-gastruloids, we modeled the effects of valproic acid (VPA) on human gastrulation and uncovered molecular pathways underlying VPA-induced malformations. When transplanted into the seminiferous tubules, hGaSCs formed embryo-like structures, progressing through fetal tissue and organ development, unlike the disorganized growth seen in teratomas. In conclusion, hGaSCs provide a versatile platform to study human gastrulation, early organogenesis, developmental defects, and drug teratogenicity, with promising applications in tissue and organ generation from cultured stem cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hoogland, S. H. A. & Marks, H. Developments in pluripotency: a new formative state. Cell Res. 31, 493–494 (2021).
Nichols, J. & Smith, A. Naive and primed pluripotent states. Cell Stem Cell 4, 487–492 (2009).
Wei, Y. et al. Dissecting embryonic and extraembryonic lineage crosstalk with stem cell co-culture. Cell 186, 5859–5875.e24 (2023).
Liu, L. et al. Modeling post-implantation stages of human development into early organogenesis with stem-cell-derived peri-gastruloids. Cell 186, 3776–3792.e16 (2023).
Chen, D. et al. Human primordial germ cells are specified from lineage-primed progenitors. Cell Rep. 29, 4568–4582.e5 (2019).
Kojima, Y. et al. Evolutionarily distinctive transcriptional and signaling programs drive human germ cell lineage specification from pluripotent stem cells. Cell Stem Cell 21, 517–532 (2017).
Xiang, L. et al. A developmental landscape of 3D-cultured human pre-gastrulation embryos. Nature 577, 537–542 (2020).
Tyser, R. C. V. et al. Single-cell transcriptomic characterization of a gastrulating human embryo. Nature 600, 285–289 (2021).
Mackinlay, K. M. et al. An in vitro stem cell model of human epiblast and yolk sac interaction. Elife 10, e63930 (2021).
Chen, L. et al. The nuclear receptor HNF4 drives a brush border gene program conserved across murine intestine, kidney, and embryonic yolk sac. Nat. Commun. 12, 2886 (2021).
Sun, S. et al. A transgene-free, human peri-gastrulation embryo model with trilaminar embryonic disc-, amnion- and yolk sac-like structures. bioRxiv https://doi.org/10.1101/2024.08.05.606556 (2024).
Consortium, E. P. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012).
Xia, W. et al. Resetting histone modifications during human parental-to-zygotic transition. Science 365, 353–360 (2019).
Zhao, C. et al. A comprehensive human embryo reference tool using single-cell RNA-sequencing data. Nat. Methods 22, 193–206 (2024).
Theunissen, T. W. et al. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 15, 471–487 (2014).
Hu, Z. et al. Transient inhibition of mTOR in human pluripotent stem cells enables robust formation of mouse-human chimeric embryos. Sci. Adv. 6, eaaz0298 (2020).
Sperber, H. et al. The metabolome regulates the epigenetic landscape during naive-to-primed human embryonic stem cell transition. Nat. Cell Biol. 17, 1523–1535 (2015).
Irie, N. et al. SOX17 is a critical specifier of human primordial germ cell fate. Cell 160, 253–268 (2015).
Takashima, Y. et al. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell 158, 1254–1269 (2014).
Yu, L. et al. Derivation of intermediate pluripotent stem cells amenable to primordial germ cell specification. Cell Stem Cell 28, 550–567.e12 (2021).
Kinoshita, M. et al. Capture of mouse and human stem cells with features of formative pluripotency. Cell Stem Cell 28, 2180 (2021).
Rostovskaya, M., Andrews, S., Reik, W. & Rugg-Gunn, P. J. Amniogenesis occurs in two independent waves in primates. Cell Stem Cell 29, 744–759 (2022).
Kobayashi, M. et al. Expanding homogeneous culture of human primordial germ cell-like cells maintaining germline features without serum or feeder layers. Stem Cell Rep. 17, 507–521 (2022).
Zheng, Y. et al. Controlled modelling of human epiblast and amnion development using stem cells. Nature 573, 421–425 (2019).
Chal, J. & Pourquié, O. Making muscle: skeletal myogenesis in vivo and in vitro. Development 144, 2104–2122 (2017).
Zhang, J. et al. Functional cardiac fibroblasts derived from human pluripotent stem cells via second heart field progenitors. Nat. Commun. 10, 2238 (2019).
Rito, T. et al. Timely TGFβ signalling inhibition induces notochord. Nature 637, 673–682 (2025).
Pedroza, M. et al. Self-patterning of human stem cells into post-implantation lineages. Nature 622, 574–583 (2023).
Karvas, R. M. et al. 3D-cultured blastoids model human embryogenesis from pre-implantation to early gastrulation stages. Cell Stem Cell 30, 1148–1165.e47 (2023).
Weatherbee, B. A. T. et al. Pluripotent stem cell-derived model of the post-implantation human embryo. Nature 622, 584–593 (2023).
Hislop, J. et al. Modelling post-implantation human development to yolk sac blood emergence. Nature 626, 367–376 (2023).
Pham, T. X. A. et al. Modeling human extraembryonic mesoderm cells using naive pluripotent stem cells. Cell Stem Cell 29, 1346–1365.e10 (2022).
Oldak, B. et al. Complete human day 14 post-implantation embryo models from naive ES cells. Nature 622, 562–573 (2023).
Kagawa, H. et al. Human blastoids model blastocyst development and implantation. Nature 601, 600–605 (2022).
Liu, X. et al. Modelling human blastocysts by reprogramming fibroblasts into iBlastoids. Nature 591, 627–632 (2021).
Yu, L. et al. Blastocyst-like structures generated from human pluripotent stem cells. Nature 591, 620–626 (2021).
Fan, Y. et al. Generation of human blastocyst-like structures from pluripotent stem cells. Cell Discov. 7, 81 (2021).
Sozen, B. et al. Reconstructing aspects of human embryogenesis with pluripotent stem cells. Nat. Commun. 12, 5550 (2021).
Ai, Z. et al. Dissecting peri-implantation development using cultured human embryos and embryo-like assembloids. Cell Res. 33, 661–678 (2023).
Zhou, F. et al. Reconstituting the transcriptome and DNA methylome landscapes of human implantation. Nature 572, 660–664 (2019).
Molè, M. A. et al. A single cell characterisation of human embryogenesis identifies pluripotency transitions and putative anterior hypoblast centre. Nat. Commun.12, 3679 (2021).
Yanagida, A. et al. Naive stem cell blastocyst model captures human embryo lineage segregation. Cell Stem Cell 28, 1016–1022.e4 (2021).
Petropoulos, S. et al. Single-cell RNA-seq reveals lineage and X chromosome dynamics in human preimplantation embryos. Cell 165, 1012–1026 (2016).
Yan, L. et al. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat. Struct. Mol. Biol. 20, 1131–1139 (2013).
Meistermann, D. et al. Integrated pseudotime analysis of human pre-implantation embryo single-cell transcriptomes reveals the dynamics of lineage specification. Cell Stem Cell 28, 1625–1640.e6 (2021).
Zeng, B. et al. The single-cell and spatial transcriptional landscape of human gastrulation and early brain development. Cell Stem Cell 30, 851–866.e7 (2023).
Nakamura, T. et al. A developmental coordinate of pluripotency among mice, monkeys and humans. Nature 537, 57–62 (2016).
Tang, X. et al. Improved in situ sequencing for high-resolution targeted spatial transcriptomic analysis in tissue sections. J. Genet. Genom. 50, 652–660 (2023).
Briscoe, J. & Thérond, P. P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 14, 416–429 (2013).
Karzbrun, E. et al. Human neural tube morphogenesis in vitro by geometric constraints. Nature 599, 268–272 (2021).
Chiu, C. T., Wang, Z., Hunsberger, J. G. & Chuang, D. M. Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol. Rev. 65, 105–142 (2013).
Balaskas, N. et al. Gene regulatory logic for reading the Sonic Hedgehog signaling gradient in the vertebrate neural tube. Cell 148, 273–284 (2012).
Gutierrez-Aranda, I. et al. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells 28, 1568–1570 (2010).
Kanatsu-Shinohara, M. et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell 119, 1001–1012 (2004).
Guan, K. et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature 440, 1199–1203 (2006).
Xu, Y. et al. A single-cell transcriptome atlas profiles early organogenesis in human embryos. Nat. Cell Biol. 25, 604–615 (2023).
Hu, Y. et al. Dissecting the transcriptome landscape of the human fetal neural retina and retinal pigment epithelium by single-cell RNA-seq analysis. PLoS Biol. 17, e3000365 (2019).
Shahbazi, M. N., Siggia, E. D. & Zernicka-Goetz, M. Self-organization of stem cells into embryos: a window on early mammalian development. Science 364, 948–951 (2019).
Martyn, I., Kanno, T. Y., Ruzo, A., Siggia, E. D. & Brivanlou, A. H. Self-organization of a human organizer by combined Wnt and Nodal signalling. Nature 558, 132–135 (2018).
Moris, N., Alev, C., Pera, M. & Martinez Arias, A. Biomedical and societal impacts of in vitro embryo models of mammalian development. Stem Cell Rep. 16, 1021–1030 (2021).
Mantziou, V. et al. In vitro teratogenicity testing using a 3D, embryo-like gastruloid system. Reprod. Toxicol. 105, 72–90 (2021).
McDonald, D. et al. Defining the teratoma as a model for multi-lineage human development. Cell 183, 1402–1419.e18 (2020).
Li, H. et al. To better generate organoids, What can we learn from teratomas? Front. Cell Dev. Biol. 9, 700482 (2021).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
He, Z., Brazovskaja, A., Ebert, S., Camp, J. G. & Treutlein, B. CSS: cluster similarity spectrum integration of single-cell genomics data. Genome Biol. 21, 224 (2020).
Jin, S., Plikus, M. V. & Nie, Q. CellChat for systematic analysis of cell-cell communication from single-cell transcriptomics. Nat. Protoc. 20, 180–219 (2025).
Acknowledgements
We thank Dangsheng Li and Yan Liu (Nanjing Medical University) for their valuable discussions and comments on our manuscript. This work was supported by the National Key R&D Program grant 2021YFC2700302 (Y.Y.), the National Natural Science Foundation of China grant 82122025 (Y.Y.), and 82221005 (J.S.), the National Key R&D Program grant 2021YFC2700200 (Y.C.), and Jiangsu Province Excellent Postdoctoral Program 2023ZB725 (M.H.).
Author information
Authors and Affiliations
Contributions
Conceptualization: J.S., Y.Y.; methodology: M.H., H.Z., G.Y., M.C., Y.C., S.Q.; investigation: M.H, H.Z., G.Y., M.C., Z.L., D.C., B.S., L.Q., J.L., L.L., J.C., Y.F.Z.; visualization: M.H., H.Z., G.Y., M.C., B.Z., Y.Y., Y.C.Z.; funding acquisition: J.S., Y.Y., Y.C., M.H.; project administration: J.S., Y.Y., J.W.; supervision: J.S., Y.Y.; writing — original draft: J.S., Y.Y., M.H., H.Z., G.Y., M.C.; writing — review & editing: J.S., J.W., Y.Y.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, M., Chen, M., Yuan, G. et al. Establishment of human gastrulating stem cells with the capacity of stable differentiation into multiple gastrulating cell types. Cell Res 35, 719–734 (2025). https://doi.org/10.1038/s41422-025-01146-z
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41422-025-01146-z
This article is cited by
-
Cultured human stem cells undergoing gastrulation
Cell Research (2025)