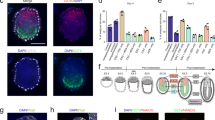
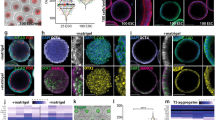
Abstract
The absence of stem cells capable of efficiently generating both trophoblast and epiblast lineages has hindered precise recapitulation of embryonic development. Through high-content chemical screening, we established an (AS and LY) AL medium to generate mouse bidirectional pluripotent stem cells (BPSCs) characterized by concurrent expression of OCT4 and CDX2. Mouse BPSCs demonstrated highly plastic differentiation into trophoblast, epiblast and primitive endoderm (PrE) lineages in vitro within 48 h without exogenous inducing factors and efficiently contributed to embryonic and extraembryonic tissues in vivo. Mechanistically, hyperactivation of the Wnt signaling pathway breaks the early lineage differentiation barrier by initiating a Lef1-dependent bypass. Remarkably, integration of BPSCs with PrE induction system enables high-efficiency generation of E8.5-stage embryo models. These advanced models complete gastrulation and recapitulate definitive developmental milestones including brain morphogenesis, neural tube closure, cardiac contraction, somite patterning, and primordial germ cell specification. Moreover, human cells cultured under AL conditions acquire an OCT4 and CDX2 double-positive state and corresponding gene expression profiles, revealing conserved functionality of this culturing platform across species. These findings highlight BPSCs as a powerful tool for investigating early lineage specification and post-gastrulation embryonic development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The bulk RNA-seq, ChIP-seq, scRNA-seq and scATAC-seq data generated during this study are available at Genome Sequence Archive of the National Genomics Data Center (https://ngdc.cncb.ac.cn/gsa/): PRJCA027661. All other data used in this study are available from the corresponding authors upon request.
References
Fleming, T. P. A quantitative analysis of cell allocation to trophectoderm and inner cell mass in the mouse blastocyst. Dev. Biol. 119, 520–531 (1987).
Dyce, J., George, M., Goodall, H. & Fleming, T. P. Do trophectoderm and inner cell mass cells in the mouse blastocyst maintain discrete lineages. Development 100, 685–698 (1987).
Johnson, M. H. & McConnell, J. M. Lineage allocation and cell polarity during mouse embryogenesis. Semin Cell Dev. Biol. 15, 583–597 (2004).
Rossant, J. & Tam, P. P. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development 136, 701–713 (2009).
Baker, C. L. & Pera, M. F. Capturing totipotent stem cells. Cell Stem Cell 22, 25–34 (2018).
Boroviak, T. & Nichols, J. Primate embryogenesis predicts the hallmarks of human naive pluripotency. Development 144, 175–186 (2017).
Tanaka, S., Kunath, T., Hadjantonakis, A. K., Nagy, A. & Rossant, J. Promotion of trophoblast stem cell proliferation by FGF4. Science 282, 2072–2075 (1998).
Evans, M. J. & Kaufman, M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 (1981).
Martin, G. R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 78, 7634–7638 (1981).
Kunath, T. et al. Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development 132, 1649–1661 (2005).
Niwa, H. et al. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell 123, 917–929 (2005).
Blij, S., Parenti, A., Tabatabai-Yazdi, N. & Ralston, A. Cdx2 efficiently induces trophoblast stem-like cells in naive, but not primed, pluripotent stem cells. Stem Cells Dev. 24, 1352–1365 (2015).
Huang, B. et al. Inhibition of HDAC activity directly reprograms murine embryonic stem cells to trophoblast stem cells. Dev. Cell 59, 2101–2117.e8 (2024).
Gao, Y. et al. Efficient reprogramming of mouse embryonic stem cells into trophoblast stem-like cells via lats kinase inhibition. Biology 13, 71 (2024).
Zhang, W. et al. Haploid-genetic screening of trophectoderm specification identifies Dyrk1a as a repressor of totipotent-like status. Sci. Adv. 9, eadi5683 (2023).
Jana, D. et al. Derivation of trophoblast stem cells unveils unrestrained potential of mouse ESCs and epiblast. Preprint at bioRxiv https://doi.org/10.1101/2023.04.19.537518 (2023).
Yang, J. et al. Establishment of mouse expanded potential stem cells. Nature 550, 393–397 (2017).
Hu, Y. et al. Induction of mouse totipotent stem cells by a defined chemical cocktail. Nature 617, 792–797 (2023).
Xu, Y. et al. Derivation of totipotent-like stem cells with blastocyst-like structure forming potential. Cell Res. 32, 513–529 (2022).
Shen, H. et al. Mouse totipotent stem cells captured and maintained through spliceosomal repression. Cell 184, 2843–2859.e20 (2021).
Peng, B., Wang, Q., Zhang, F., Shen, H. & Du, P. Mouse totipotent blastomere-like cells model embryogenesis from zygotic genome activation to post implantation. Cell Stem Cell 32, 391–408.e11 (2025).
Posfai, E. et al. Evaluating totipotency using criteria of increasing stringency. Nat. Cell Biol. 23, 49–60 (2021).
Tarazi, S. et al. Post-gastrulation synthetic embryos generated ex utero from mouse naive ESCs. Cell 185, 3290–3306.e25 (2022).
Amadei, G. et al. Embryo model completes gastrulation to neurulation and organogenesis. Nature 610, 143–153 (2022).
Sozen, B. et al. Self-assembly of embryonic and two extra-embryonic stem cell types into gastrulating embryo-like structures. Nat. Cell Biol. 20, 979–989 (2018).
Rivron, N. C. et al. Blastocyst-like structures generated solely from stem cells. Nature 557, 106–111 (2018).
Li, R. et al. Generation of blastocyst-like structures from mouse embryonic and adult cell cultures. Cell 179, 687–702.e18 (2019).
Sozen, B. et al. Self-organization of mouse stem cells into an extended potential blastoid. Dev. Cell 51, 698–712.e8 (2019).
Sozen, B. et al. Self-assembly of embryonic and two extra- embryonic stem cell types into gastrulating embryo-like structures. Nat. Cell Biol. 20, 979–989 (2018).
Veenvliet, J. V. et al. Mouse embryonic stem cells self-organize into trunk-like structures with neural tube and somites. Science 370, eaba4937 (2020).
Harrison, S. E., Sozen, B., Christodoulou, N., Kyprianou, C. & Zernicka-Goetz, M. Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science 356, eaal1810 (2017).
Beccari, L. et al. Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature 562, 272–276 (2018).
Dietrich, J. E. & Hiiragi, T. Stochastic patterning in the mouse pre-implantation embryo. Development 134, 4219–4231 (2007).
Suwinska, A., Czolowska, R., Ozdzenski, W. & Tarkowski, A. K. Blastomeres of the mouse embryo lose totipotency after the fifth cleavage division: expression of Cdx2 and Oct4 and developmental potential of inner and outer blastomeres of 16- and 32-cell embryos. Dev. Biol. 322, 133–144 (2008).
Mihajlovic, A. I. & Bruce, A. W. The first cell-fate decision of mouse preimplantation embryo development: integrating cell position and polarity. Open Biol. 7, 170210 (2017).
Tarkowski, A. K., Suwinska, A., Czolowska, R. & Ozdzenski, W. Individual blastomeres of 16- and 32-cell mouse embryos are able to develop into foetuses and mice. Dev. Biol. 348, 190–198 (2010).
Gao, X. et al. Establishment of porcine and human expanded potential stem cells. Nat. Cell Biol. 21, 687–699 (2019).
Nowotschin, S. et al. The emergent landscape of the mouse gut endoderm at single-cell resolution. Nature 569, 361–367 (2019).
Atlasi, Y. et al. Wnt signaling regulates the lineage differentiation potential of mouse embryonic stem cells through Tcf3 down-regulation. PLoS Genet. 9, e1003424 (2013).
Shy, B. R. et al. Regulation of Tcf7l1 DNA binding and protein stability as principal mechanisms of Wnt/beta-catenin signaling. Cell Rep. 4, 1–9 (2013).
He, S., Pant, D., Schiffmacher, A., Meece, A. & Keefer, C. L. Lymphoid enhancer factor 1-mediated Wnt signaling promotes the initiation of trophoblast lineage differentiation in mouse embryonic stem cells. Stem Cells 26, 842–849 (2008).
Ma, H. et al. In vitro culture of cynomolgus monkey embryos beyond early gastrulation. Science 366, eaax7890 (2019).
Aguilera-Castrejon, A. et al. Ex utero mouse embryogenesis from pre-gastrulation to late organogenesis. Nature 593, 119–124 (2021).
Theunissen, T. W. et al. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 15, 471–487 (2014).
Yan, L. et al. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat. Struct. Mol. Biol. 20, 1131–1139 (2013).
Petropoulos, S. et al. Single-cell RNA-seq reveals lineage and X chromosome dynamics in human preimplantation embryos. Cell 167, 285 (2016).
Xiang, L. et al. A developmental landscape of 3D-cultured human pre-gastrulation embryos. Nature 577, 537–542 (2020).
Tyser, R. C. V. et al. Single-cell transcriptomic characterization of a gastrulating human embryo. Nature 600, 285–289 (2021).
Meistermann, D. et al. Integrated pseudotime analysis of human pre-implantation embryo single-cell transcriptomes reveals the dynamics of lineage specification. Cell Stem Cell 28, 1625–1640.e6 (2021).
Yanagida, A. et al. Naive stem cell blastocyst model captures human embryo lineage segregation. Cell Stem Cell 28, 1016–1022.e4 (2021).
Ziomek, C. A., Johnson, M. H. & Handyside, A. H. The developmental potential of mouse 16-cell blastomeres. J. Exp. Zool. 221, 345–355 (1982).
Chen, Y., Blair, K. & Smith, A. Robust self-renewal of rat embryonic stem cells requires fine-tuning of glycogen synthase kinase-3 inhibition. Stem Cell Rep. 1, 209–217 (2013).
Zhang, X. et al. FOXO1 is an essential regulator of pluripotency in human embryonic stem cells. Nat. Cell Biol. 13, 1092–1099 (2011).
Jeon, J. H., Suh, H. N., Kim, M. O., Ryu, J. M. & Han, H. J. Glucosamine-induced OGT activation mediates glucose production through cleaved Notch1 and FoxO1, which coordinately contributed to the regulation of maintenance of self-renewal in mouse embryonic stem cells. Stem Cells Dev. 23, 2067–2079 (2014).
Szabo, P. E., Hubner, K., Scholer, H. & Mann, J. R. Allele-specific expression of imprinted genes in mouse migratory primordial germ cells. Mech. Dev. 115, 157–160 (2002).
Zhang, W. et al. A high-throughput small molecule screen identifies farrerol as a potentiator of CRISPR/Cas9-mediated genome editing. Elife 9, e56008 (2020).
Bi, Y. et al. Identification of ALPPL2 as a naive pluripotent state-specific surface protein essential for human naive pluripotency regulation. Cell Rep. 30, 3917–3931.e5 (2020).
Bi, Y. et al. Cell fate roadmap of human primed-to-naive transition reveals preimplantation cell lineage signatures. Nat. Commun. 13, 3147 (2022).
Pastor, W. A. et al. Naive human pluripotent cells feature a methylation landscape devoid of blastocyst or germline memory. Cell Stem Cell 18, 323–329 (2016).
Mazid, M. A. et al. Rolling back human pluripotent stem cells to an eight-cell embryo-like stage. Nature 605, 315–324 (2022).
Yang, Y. et al. Derivation of pluripotent stem cells with in vivo embryonic and extraembryonic potency. Cell 169, 243–257.e25 (2017).
Zhou, Y. Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019).
Stuart, T., Srivastava, A., Madad, S., Lareau, C. A. & Satija, R. Single-cell chromatin state analysis with Signac. Nat. Methods 18, 1333–1341 (2021).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Nakato, R. & Sakata, T. Methods for ChIP-seq analysis: a practical workflow and advanced applications. Methods 187, 44–53 (2021).
Bailey, T. et al. Practical guidelines for the comprehensive analysis of ChIP-seq data. PLoS Comput. Biol. 9, e1003326 (2013).
Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv https://doi.org/10.48550/arXiv.1303.3997 (2013).
Ramirez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Amemiya, H. M., Kundaje, A. & Boyle, A. P. The ENCODE blacklist: identification of problematic regions of the genome. Sci. Rep. 9, 9354 (2019).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Wu, J. et al. The landscape of accessible chromatin in mammalian preimplantation embryos. Nature 534, 652–657 (2016).
Wu, Y. et al. N(6)-methyladenosine regulates maternal RNA maintenance in oocytes and timely RNA decay during mouse maternal-to-zygotic transition. Nat. Cell Biol. 24, 917–927 (2022).
Deng, Q., Ramskold, D., Reinius, B. & Sandberg, R. Single-cell RNA-seq reveals dynamic, random monoallelic gene expression in mammalian cells. Science 343, 193–196 (2014).
Posfai, E. et al. Position- and Hippo signaling-dependent plasticity during lineage segregation in the early mouse embryo. Elife 6, e22906 (2017).
Li, M. et al. Chromatin accessibility landscape of mouse early embryos revealed by single-cell NanoATAC-seq2. Science 387, eadp4319 (2025).
Imaz-Rosshandler, I. et al. Tracking early mammalian organogenesis - prediction and validation of differentiation trajectories at whole organism scale. Development 151, dev201867 (2024).
Liu, K. et al. Bilineage embryo-like structure from EPS cells can produce live mice with tetraploid trophectoderm. Protein Cell 14, 262–278 (2023).
Wu, K. et al. Dynamics of histone acetylation during human early embryogenesis. Cell Discov. 9, 29 (2023).
Acknowledgements
This work was primarily supported by the National Key R&D Program of China (2021YFA1102900) and the National Natural Science Foundation of China (32488101). This work was also supported by the National Key R&D Program of China (2022YFC2702200 and 2024YFA1107000) and the National Natural Science Foundation of China (32330030, 82301921, 32070823, 92168205, 32270909, 82201881, 92168205, 31871448, 31820103009, 82022027, 32300684, 32400677 and 32370842). This work was also supported by the Key Project of the Science and Technology of Shanghai Municipality (19JC1415300 and 21JC1405500), the Natural Science Foundation of Shanghai (21ZR1450700), China Postdoctoral Science Foundation (2023M732660, 2023M732661), the Postdoctoral Fellowship Program of China Postdoctoral Science Foundation (GZB20230523), Shanghai Pilot Program for Basic Research, Shanghai Post-doctoral Excellence Program (2022521, 2022551), Shenzhen Medical Research Fund (B2402013), the Fundamental Research Funds for the Central Universities (22120250374), Peak Disciplines (Type IV) of Institutions of Higher Learning in Shanghai and Ministry of Education. We thank Rongrong Le (Tongji University), leader of the ethical approval project (2024tjdxsy026), to provide the human cell lines in this study.
Author information
Authors and Affiliations
Contributions
S.G., W.L. and Y.W. conceived the project and provided mentoring; K.L., D.B., Y.B. and X.M. designed and performed most of the experiments; Z.Y. provided bioinformatics support; R.J. and K.W. performed compound library screening; K.L., D.B. and Z.Y. wrote the original draft; K.L., D.B., Z.Y., W.L., J.Y. and S.G. reviewed and edited the manuscript; J.X., Y.S., B.D., Z.N., S.Y., Y.D.L., X.L., Y.J., Y.Z., Y.L.Z., Y.H.L., T.W., C.X., Shanyao L., Shuyi L., J.C., J.Y., X.K., Y.H.Z. and H.W. assisted with the experiments.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, K., Yan, Z., Bai, D. et al. Modeling post-gastrula development via bidirectional pluripotent stem cells. Cell Res 35, 954–969 (2025). https://doi.org/10.1038/s41422-025-01172-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41422-025-01172-x