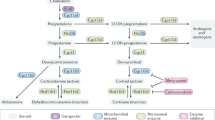
Abstract
Glucocorticoids (GCs) are endogenous hormones that are crucial for the homeostasis of the organism and adaptation to the external environment. Because of their anti-inflammatory effects, synthetic GCs are also extensively used in clinical practice. However, almost all cells in the body are sensitive to GC regulation. As a result, these mediators have pleiotropic effects, which may be undesirable or detrimental to human health. Here, we summarize the recent findings that contribute to deciphering the molecular mechanisms downstream of glucocorticoid receptor activation. We also discuss the complex role of GCs in infectious diseases such as sepsis and COVID-19, in which the balance between pathogen elimination and protection against excessive inflammation and immunopathology needs to be tightly regulated. An understanding of the cell type- and context-specific actions of GCs from the molecular to the organismal level would help to optimize their therapeutic use.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Oster, H. et al. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr. Rev. 38, 3–45 (2017).
Cole, T. J. et al. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 9, 1608–1621 (1995).
Vandewalle, J., Luypaert, A., De Bosscher, K. & Libert, C. Therapeutic mechanisms of glucocorticoids. Trends Endocrinol. Metab. 29, 42–54 (2018).
Frei, E. 3rd et al. The effectiveness of combinations of antileukemic agents in inducing and maintaining remission in children with acute leukemia. Blood 26, 642–656 (1965).
Herr, I. & Pfitzenmaier, J. Glucocorticoid use in prostate cancer and other solid tumours: implications for effectiveness of cytotoxic treatment and metastases. Lancet Oncol. 7, 425–430 (2006).
Holgate, S. T. & Polosa, R. Treatment strategies for allergy and asthma. Nat. Rev. Immunol. 8, 218–230 (2008).
Dendoncker, K. & Libert, C. Glucocorticoid resistance as a major drive in sepsis pathology. Cytokine growth factor Rev. 35, 85–96 (2017).
Galon, J. et al. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 16, 61–71 (2002).
Cain, D. W. & Cidlowski, J. A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 17, 233–247 (2017).
Kadmiel, M. & Cidlowski, J. A. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol. Sci. 34, 518–530 (2013).
Meijsing, S. H. et al. DNA binding site sequence directs glucocorticoid receptor structure and activity. Science 324, 407–410 (2009).
Hudson, W. H., Youn, C. & Ortlund, E. A. The structural basis of direct glucocorticoid-mediated transrepression. Nat. Struct. Mol. Biol. 20, 53–58 (2013).
Timmermans, S., Souffriau, J. & Libert, C. A general introduction to glucocorticoid biology. Front. Immunol. 10, 1545 (2019).
Rhen, T. & Cidlowski, J. A. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N. Engl. J. Med. 353, 1711–1723 (2005).
Kassel, O. et al. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 20, 7108–7116 (2001).
Lasa, M., Abraham, S. M., Boucheron, C., Saklatvala, J. & Clark, A. R. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol. Cell. Biol. 22, 7802–7811 (2002).
Widen, C., Gustafsson, J. A. & Wikstrom, A. C. Cytosolic glucocorticoid receptor interaction with nuclear factor-kappa B proteins in rat liver cells. Biochemical J. 373, 211–220 (2003).
De Bosscher, K. et al. Glucocorticoids repress NF-kappaB-driven genes by disturbing the interaction of p65 with the basal transcription machinery, irrespective of coactivator levels in the cell. Proc. Natl Acad. Sci. USA 97, 3919–3924 (2000).
De Bosscher, K., Vanden Berghe, W. & Haegeman, G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr. Rev. 24, 488–522 (2003).
Berrebi, D. et al. Synthesis of glucocorticoid-induced leucine zipper (GILZ) by macrophages: an anti-inflammatory and immunosuppressive mechanism shared by glucocorticoids and IL-10. Blood 101, 729–738 (2003).
Oakley, R. H. & Cidlowski, J. A. Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids. J. Biol. Chem. 286, 3177–3184 (2011).
John, S. et al. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat. Genet. 43, 264–268 (2011).
Franco, L. M. et al. Immune regulation by glucocorticoids can be linked to cell type-dependent transcriptional responses. J. Exp. Med. 216, 384–406 (2019).
Dejager, L. et al. Neutralizing TNFalpha restores glucocorticoid sensitivity in a mouse model of neutrophilic airway inflammation. Mucosal Immunol. 8, 1212–1225 (2015).
Van Bogaert, T. et al. Tumor necrosis factor inhibits glucocorticoid receptor function in mice: a strong signal toward lethal shock. J. Biol. Chem. 286, 26555–26567 (2011).
Dendoncker, K. et al. TNF-alpha inhibits glucocorticoid receptor-induced gene expression by reshaping the GR nuclear cofactor profile. Proc. Natl Acad. Sci. USA 116, 12942–12951 (2019).
Reichardt, H. M. et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell 93, 531–541 (1998).
Whirledge, S. & DeFranco, D. B. Glucocorticoid signaling in health and disease: insights from tissue-specific GR knockout mice. Endocrinology 159, 46–64 (2018).
Wyllie, A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284, 555–556 (1980).
Strehl, C., Ehlers, L., Gaber, T. & Buttgereit, F. Glucocorticoids-all-rounders tackling the versatile players of the immune system. Front. Immunol. 10, 1744 (2019).
Zacharchuk, C. M., Mercep, M., Chakraborti, P. K., Simons, S. S. Jr. & Ashwell, J. D. Programmed T lymphocyte death. Cell activation- and steroid-induced pathways are mutually antagonistic. J. Immunol. 145, 4037–4045 (1990).
Liberman, A. C. et al. The activated glucocorticoid receptor inhibits the transcription factor T-bet by direct protein-protein interaction. FASEB J. 21, 1177–1188 (2007).
Jee, Y. K. et al. Repression of interleukin-5 transcription by the glucocorticoid receptor targets GATA3 signaling and involves histone deacetylase recruitment. J. Biol. Chem. 280, 23243–23250 (2005).
Maneechotesuwan, K. et al. Suppression of GATA-3 nuclear import and phosphorylation: a novel mechanism of corticosteroid action in allergic disease. PLoS Med. 6, e1000076 (2009).
Liberman, A. C., Druker, J., Refojo, D., Holsboer, F. & Arzt, E. Glucocorticoids inhibit GATA-3 phosphorylation and activity in T cells. FASEB J. 23, 1558–1571 (2009).
Hu, C. et al. Glucocorticoids modulate Th1 and Th2 responses in asthmatic mouse models by inhibition of Notch1 signaling. Int. Arch. allergy Immunol. 175, 44–52 (2018).
Elenkov, I. J. Glucocorticoids and the Th1/Th2 balance. Ann. N. Y. Acad. Sci. 1024, 138–146 (2004).
Banuelos, J., Cao, Y., Shin, S. C. & Lu, N. Z. Immunopathology alters Th17 cell glucocorticoid sensitivity. Allergy 72, 331–341 (2017).
Shimba, A. et al. Glucocorticoids drive diurnal oscillations in T cell distribution and responses by inducing interleukin-7 receptor and CXCR4. Immunity 48, 286–298.e6 (2018).
Cain, D. W. et al. Murine glucocorticoid receptors orchestrate B cell migration selectively between bone marrow and blood. J. Immunol. 205, 619–629 (2020).
Burger, J. A. & Montserrat, E. Coming full circle: 70 years of chronic lymphocytic leukemia cell redistribution, from glucocorticoids to inhibitors of B-cell receptor signaling. Blood 121, 1501–1509 (2013).
Quatrini, L. et al. Endogenous glucocorticoids control host resistance to viral infection through the tissue-specific regulation of PD-1 expression on NK cells. Nat. Immunol. 19, 954–962 (2018).
Quatrini, L. et al. Glucocorticoids and the cytokines IL-12, IL-15 and IL-18 present in the tumor microenvironment induce PD-1 expression on human Natural Killer cells. J. Allergy clinical Immunol. https://doi.org/10.1016/j.jaci.2020.04.044 (2020).
Xing, K., Gu, B., Zhang, P. & Wu, X. Dexamethasone enhances programmed cell death 1 (PD-1) expression during T cell activation: an insight into the optimum application of glucocorticoids in anti-cancer therapy. BMC Immunol. 16, 39 (2015).
Maeda, N. et al. Glucocorticoids potentiate the inhibitory capacity of programmed cell death 1 by up-regulating its expression on T cells. J. Biol. Chem. 294, 19896–19906 (2019).
Tokunaga, A. et al. Selective inhibition of low-affinity memory CD8(+) T cells by corticosteroids. J. Exp. Med. 216, 2701–2713 (2019).
Rudak, P. T. et al. Stress-elicited glucocorticoid receptor signaling upregulates TIGIT in innate-like invariant T lymphocytes. Brain, Behav., Immun. 80, 793–804 (2019).
Ma, W. et al. Dexamethasone inhibits IL-12p40 production in lipopolysaccharide-stimulated human monocytic cells by down-regulating the activity of c-Jun N-terminal kinase, the activation protein-1, and NF-kappa B transcription factors. J. Immunol. 172, 318–330 (2004).
Larsson, S. & Linden, M. Effects of a corticosteroid, budesonide, on production of bioactive IL-12 by human monocytes. Cytokine 10, 786–789 (1998).
Visser, J. et al. Differential regulation of interleukin-10 (IL-10) and IL-12 by glucocorticoids in vitro. Blood 91, 4255–4264 (1998).
Di Rosa, M., Radomski, M., Carnuccio, R. & Moncada, S. Glucocorticoids inhibit the induction of nitric oxide synthase in macrophages. Biochem. Biophys. Res. Commun. 172, 1246–1252 (1990).
Lee, S. H. et al. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J. Biol. Chem. 267, 25934–25938 (1992).
Abraham, S. M. et al. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J. Exp. Med. 203, 1883–1889 (2006).
Pfander, P., Fidan, M., Burret, U., Lipinski, L. & Vettorazzi, S. Cdk5 deletion enhances the anti-inflammatory potential of GC-mediated GR activation during inflammation. Front. Immunol. 10, 1554 (2019).
Tuckermann, J. P. et al. Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J. Clin. Investig. 117, 1381–1390 (2007).
Bhattacharyya, S., Brown, D. E., Brewer, J. A., Vogt, S. K. & Muglia, L. J. Macrophage glucocorticoid receptors regulate Toll-like receptor 4-mediated inflammatory responses by selective inhibition of p38 MAP kinase. Blood 109, 4313–4319 (2007).
Kleiman, A. et al. Glucocorticoid receptor dimerization is required for survival in septic shock via suppression of interleukin-1 in macrophages. FASEB J. 26, 722–729 (2012).
Yang, H. et al. Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced antitumor immunity. Nat. Med. 25, 1428–1441 (2019).
Hermoso, M. A., Matsuguchi, T., Smoak, K. & Cidlowski, J. A. Glucocorticoids and tumor necrosis factor alpha cooperatively regulate toll-like receptor 2 gene expression. Mol. Cell. Biol. 24, 4743–4756 (2004).
Lannan, E. A., Galliher-Beckley, A. J., Scoltock, A. B. & Cidlowski, J. A. Proinflammatory actions of glucocorticoids: glucocorticoids and TNFalpha coregulate gene expression in vitro and in vivo. Endocrinology 153, 3701–3712 (2012).
Mattiola, I. et al. The macrophage tetraspan MS4A4A enhances dectin-1-dependent NK cell-mediated resistance to metastasis. Nat. Immunol. 20, 1012–1022 (2019).
Irwin, M. R. & Cole, S. W. Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol. 11, 625–632 (2011).
Quatrini, L., Vivier, E. & Ugolini, S. Neuroendocrine regulation of innate lymphoid cells. Immunological Rev. 286, 120–136 (2018).
Ehrchen, J. et al. Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood 109, 1265–1274 (2007).
Mozo, L., Suarez, A. & Gutierrez, C. Glucocorticoids up-regulate constitutive interleukin-10 production by human monocytes. Clin. Exp. Allergy 34, 406–412 (2004).
Tu, G. W. et al. Glucocorticoid attenuates acute lung injury through induction of type 2 macrophage. J. Transl. Med. 15, 181 (2017).
Meers, G. K., Bohnenberger, H., Reichardt, H. M., Luhder, F. & Reichardt, S. D. Impaired resolution of DSS-induced colitis in mice lacking the glucocorticoid receptor in myeloid cells. PLoS ONE 13, e0190846 (2018).
Buechler, C. et al. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J. Leukoc. Biol. 67, 97–103 (2000).
Vallelian, F. et al. Glucocorticoid treatment skews human monocyte differentiation into a hemoglobin-clearance phenotype with enhanced heme-iron recycling and antioxidant capacity. Blood 116, 5347–5356 (2010).
Giles, K. M. et al. Glucocorticoid augmentation of macrophage capacity for phagocytosis of apoptotic cells is associated with reduced p130Cas expression, loss of paxillin/pyk2 phosphorylation, and high levels of active Rac. J. Immunol. 167, 976–986 (2001).
van der Goes, A., Hoekstra, K., van den Berg, T. K. & Dijkstra, C. D. Dexamethasone promotes phagocytosis and bacterial killing by human monocytes/macrophages in vitro. J. Leukoc. Biol. 67, 801–807 (2000).
Le Tulzo, Y. et al. Monocyte human leukocyte antigen-DR transcriptional downregulation by cortisol during septic shock. Am. J. Respir. Crit. Care Med. 169, 1144–1151 (2004).
Celada, A., McKercher, S. & Maki, R. A. Repression of major histocompatibility complex IA expression by glucocorticoids: the glucocorticoid receptor inhibits the DNA binding of the X box DNA binding protein. J. Exp. Med. 177, 691–698 (1993).
Yona, S. & Gordon, S. Inflammation: glucocorticoids turn the monocyte switch. Immunol. Cell Biol. 85, 81–82 (2007).
Cecconi, M., Evans, L., Levy, M. & Rhodes, A. Sepsis and septic shock. Lancet 392, 75–87 (2018).
Perlstein, R. S., Whitnall, M. H., Abrams, J. S., Mougey, E. H. & Neta, R. Synergistic roles of interleukin-6, interleukin-1, and tumor necrosis factor in the adrenocorticotropin response to bacterial lipopolysaccharide in vivo. Endocrinology 132, 946–952 (1993).
Rivier, C., Chizzonite, R. & Vale, W. In the mouse, the activation of the hypothalamic-pituitary-adrenal axis by a lipopolysaccharide (endotoxin) is mediated through interleukin-1. Endocrinology 125, 2800–2805 (1989).
Ramachandra, R. N., Sehon, A. H. & Berczi, I. Neuro-hormonal host defence in endotoxin shock. Brain, Behav., Immun. 6, 157–169 (1992).
Cavaillon, J. M. & Adib-Conquy, M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit. Care 10, 233 (2006).
Adib-Conquy, M. et al. Up-regulation of MyD88s and SIGIRR, molecules inhibiting Toll-like receptor signaling, in monocytes from septic patients. Crit. Care Med. 34, 2377–2385 (2006).
Yoza, B. K. & McCall, C. E. Facultative heterochromatin formation at the IL-1 beta promoter in LPS tolerance and sepsis. Cytokine 53, 145–152 (2011).
Foster, S. L., Hargreaves, D. C. & Medzhitov, R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447, 972–978 (2007).
Porta, C. et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc. Natl Acad. Sci. USA 106, 14978–14983 (2009).
Lopez-Collazo, E. & del Fresno, C. Pathophysiology of endotoxin tolerance: mechanisms and clinical consequences. Crit. Care 17, 242 (2013).
Quatrini, L. et al. Host resistance to endotoxic shock requires the neuroendocrine regulation of group 1 innate lymphoid cells. J. Exp. Med. 214, 3531–3541 (2017).
Li, C. C., Munitic, I., Mittelstadt, P. R., Castro, E. & Ashwell, J. D. Suppression of dendritic cell-derived IL-12 by endogenous glucocorticoids is protective in LPS-induced sepsis. PLoS Biol. 13, e1002269 (2015).
Sprung, C. L. et al. The effects of high-dose corticosteroids in patients with septic shock. A prospective, controlled study. N. Engl. J. Med. 311, 1137–1143 (1984).
Bone, R. C. et al. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N. Engl. J. Med. 317, 653–658 (1987).
Annane, D. et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 288, 862–871 (2002).
Sprung, C. L. et al. Hydrocortisone therapy for patients with septic shock. N. Engl. J. Med. 358, 111–124 (2008).
Venkatesh, B. et al. Adjunctive glucocorticoid therapy in patients with septic shock. N. Engl. J. Med. 378, 797–808 (2018).
Annane, D. et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N. Engl. J. Med. 378, 809–818 (2018).
Rhodes, A. et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 43, 304–377 (2017).
Biswas, S. K. & Lopez-Collazo, E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 30, 475–487 (2009).
Brandish, P. E. et al. Development of anti-CD74 antibody-drug conjugates to target glucocorticoids to immune cells. Bioconjugate Chem. 29, 2357–2369 (2018).
Li, H. et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet 395, 1517–1520 (2020).
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020).
Chen, G. et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 130, 2620–2629 (2020).
Ledford, H. How does COVID-19 kill? Uncertainty is hampering doctors’ ability to choose treatments. Nature 580, 311–312 (2020).
Metselaar, J. M., Wauben, M. H., Wagenaar-Hilbers, J. P., Boerman, O. C. & Storm, G. Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating liposomes. Arthritis Rheum. 48, 2059–2066 (2003).
Metselaar, J. M. et al. Liposomal targeting of glucocorticoids to synovial lining cells strongly increases therapeutic benefit in collagen type II arthritis. Ann. Rheum. Dis. 63, 348–353 (2004).
Oikarinen, A. & Autio, P. New aspects of the mechanism of corticosteroid-induced dermal atrophy. Clin. Exp. Dermatol. 16, 416–419 (1991).
Oikarinen, A., Haapasaari, K. M., Sutinen, M. & Tasanen, K. The molecular basis of glucocorticoid-induced skin atrophy: topical glucocorticoid apparently decreases both collagen synthesis and the corresponding collagen mRNA level in human skin in vivo. Br. J. Dermatol. 139, 1106–1110 (1998).
Perez, P. et al. Altered skin development and impaired proliferative and inflammatory responses in transgenic mice overexpressing the glucocorticoid receptor. FASEB J. 15, 2030–2032 (2001).
Beer, H. D., Fassler, R. & Werner, S. Glucocorticoid-regulated gene expression during cutaneous wound repair. Vitam. Hormones. 59, 217–239 (2000).
Skoner, D. P. et al. Detection of growth suppression in children during treatment with intranasal beclomethasone dipropionate. Pediatrics 105, E23 (2000).
Reid, I. R. Glucocorticoid-induced osteoporosis. Bailliere’s Best. Pract. Res. Clin. Endocrinol. Metab. 14, 279–298 (2000).
Barnes, P. J. & Adcock, I. M. Glucocorticoid resistance in inflammatory diseases. Lancet 373, 1905–1917 (2009).
Schacke, H., Docke, W. D. & Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Therapeutics 96, 23–43 (2002).
Schacke, H. et al. Dissociation of transactivation from transrepression by a selective glucocorticoid receptor agonist leads to separation of therapeutic effects from side effects. Proc. Natl Acad. Sci. USA 101, 227–232 (2004).
Coghlan, M. J. et al. A novel antiinflammatory maintains glucocorticoid efficacy with reduced side effects. Mol. Endocrinol. 17, 860–869 (2003).
Klassen, C. et al. Airway epithelial cells are crucial targets of glucocorticoids in a mouse model of allergic asthma. J. Immunol. 199, 48–61 (2017).
Obradovic, M. M. S. et al. Glucocorticoids promote breast cancer metastasis. Nature 567, 540–544 (2019).
Cui, B. et al. Targeting ROR1 inhibits epithelial-mesenchymal transition and metastasis. Cancer Res. 73, 3649–3660 (2013).
Acknowledgements
The SU laboratory received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Program under grant agreement no. 648768, the Agence Nationnale de la Recherche (ANR) (No. ANR-14-CE14-0009-01), and the ARC foundation (No. PGA120140200817). The SU laboratory is also supported by institutional grants from INSERM, CNRS, Aix-Marseille University and Marseille-Immunopole to the CIML. L.Q. has received funding from AIRC and from the European Union’s Horizon 2020 Research and Innovation Program under the Marie Skłodowska-Curie grant agreement no. 800924.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Quatrini, L., Ugolini, S. New insights into the cell- and tissue-specificity of glucocorticoid actions. Cell Mol Immunol 18, 269–278 (2021). https://doi.org/10.1038/s41423-020-00526-2
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41423-020-00526-2
Keywords
This article is cited by
-
A cross-tissue transcriptomic approach decodes glucocorticoid receptor-dependent links to human metabolic phenotypes
BMC Genomics (2025)
-
Impact of intraoperative furosemide and dexamethasone on complications following mini-percutaneous nephrolithotripsy: a retrospective propensity score-matched cohort study
BMC Urology (2025)
-
A double-edged sword: a narrative review on steroids in sepsis and septic shock
Internal and Emergency Medicine (2025)
-
Glucocorticoids increase adiposity by stimulating Krüppel-like factor 9 expression in macrophages
Nature Communications (2024)
-
Association of cumulative methylprednisolone dosages with mortality risk from pneumonia in connective tissue disease patients
Scientific Reports (2024)